ARTICULO ORIGINAL
DOI 10.25176/RFMH.v19.n1.1788FACTORS ASSOCIATED WITH PROGNOSIS AND SURVIVAL OF HOSPITALIZED ADULT PATIENTS WITH ACUTE MYELOID LEUKEMIA DIAGNOSIS AT HOSPITAL NACIONAL DOS DE MAYO TERM FROM 2014 TO 2016
FACTORES ASOCIADOS AL PRONÓSTICO Y SUPERVIVENCIA DE PACIENTES ADULTOS HOSPITALIZADOS CON DIAGNÓSTICO DE LEUCEMIA MIELOIDE AGUDA DEL HOSPITAL NACIONAL DOS DE MAYO PERÍODO 2014 A 2016
Nicolás J. Higueras-Bromley1,a, Luis A. Cano-Cárdenas2, Nancy E. Loayza-Urcia2, Jhony A. De La Cruz-Vargas3
1Hospital Nacional Dos de Mayo, Lima-Peru 2Hospital Nacional Dos de Mayo, Lima-Peru. 3Institute of Research in Biomedical Sciences, Universidad Ricardo Palma, Lima - Peru. aMedical Intern.
ABSTRACT:
Introduction:
Survival in patients diagnosed with acute myeloid leukemia (AML) may be affected by various clinical,
cytogenetic and immunophenotypic factors.
Methods:
An observational, transversal, retrospective and
analytical study was conducted, based on the review of clinical histories of all adult patients with
diagnosis of acute myeloid leukemia diagnosed in April 2014 to December 2016, a follow-up was made to
determine their final outcome at 2 years. We calculated the OR and performed survival curves of each
prognosis variable independent associated with survival in 3 times: 6 months, 1 year and 2 years.
Results:
Of
the 39 patients 16 (41%) were older than 60 years, 23 (59%) were less than 60 years, 13 of the patients (33%)
showed a number of leukocytes greater than 50 000 to the diagnosis and 26 (77%) showed a number less than 50
000. 33 patients presented a De Novo leukemia (84%) and 6 (16 %) presented leukemia secondary to a myelo
dysplastic syndrome or some antecedent of previous chemotherapy. No complete data were obtained from all
patients according to FAB classification, Immunophenotyping and Karyotype. Survival was assessed in 3 times,
at 6 months, year and 2 years from the date of diagnosis, overall survival of the 39 patients was 69% (25) at
6 months, and 49% (18) at 1 and 2 years. For the survival of the year and 2 years of the diagnosis we got the
same OR of 4.5 for the variable leukocytes to the diagnosis greater than 50 000(IC 95%: 1,008 – 20,507),
considering it as a risk factor for mortality. In the survival analysis of the same variable mentioned,
survival at year and two years was significantly lower for the group of patients with leukocytes at diagnosis
greater than 50 000.
Conclusion:
In our study, statistical significance was found evaluating the variable
Leukocytes at diagnosis greater than 50 000, finding it as an associated factor with mortality to 2 years,
with a significantly greater survival than the group of patients with leukocytes at diagnosis less than 50
000.
Key words:
Acute myeloid leukemia; Prognosis; Survival. (source: MeSH NLM)
RESUMEN:
Introduccion:
La supervivencia en pacientes con diagnóstico de Leucemia Mieloide Aguda (LMA) se puede ver afectada por
diversos factores clínicos, citogenéticos e inmunofenotípicos.
Objetivo:
Determinar las características del
tamizaje para cáncer CCU en 08 establecimientos de salud (EESS) de Lima Metropolitana sedes del Internado
Médico de la FAMURP en el 2017.
Métodos:
Se realizó un estudio observacional,
transversal, retrospectivo y
analítico, basado en la revisión de historias clínicas de todos los pacientes adultos con diagnóstico de
Leucemia Mieloide Aguda, diagnosticados en Abril del 2014 a diciembre de 2016 y se realizó un seguimiento
para determinar su desenlace final a 2 años asociándolo a la presencia de factores condiciones de mortalidad.
La muestra final fue de 39 pacientes.
Resultados:
De los 39 pacientes 16 (41 %)
fueron mayores de 60 años, 23
(59 %) fueron menores de 60 años, 13 de los pacientes (33 %) presentaron una cifra de Leucocitos mayor a 50
000 al diagnóstico y 26 (77 %) presentaron una cifra menor a 50 000. 33 pacientes presentaron una Leucemia de
Novo (84 %) y 6 (16 %) presentaron una Leucemia secundaria a un Síndrome Mielo Displásico o algún antecedente
de quimioterapia previa. No se obtuvo datos de todos los pacientes según clasificación Franco Americano
Británica (FAB), Inmunofenotipo y Cariotipo. La supervivencia se evaluó en 3 tiempos, a los 6 meses, al año y
a los 2 años desde la fecha del diagnóstico, teniendo que a los 6 meses la sobrevida global de los 39
pacientes había sido el 69 % (25), al año 49 % (18) y a los 2 años se mantuvo en el mismo rango. La
supervivencia al año y 2 años del diagnóstico se obtuvo el mismo Odds Ratio (OR) de 4.5 para la variable
Leucocitos al diagnóstico mayor a 50 000 (IC 95 %: 1.008 – 20.507), considerándola como un factor de riesgo
para mortalidad. En el análisis de supervivencia de la misma variable mencionada con la supervivencia al año
y dos años que la supervivencia fue significativamente menor en el grupo de pacientes con leucocitos al
diagnóstico mayor a 50 000.
Conclusión:
En nuestra población de estudio se encontró
significancia estadística
al momento de evaluar la variable Leucocitos al diagnóstico mayor a 50 000 encontrándola como factor asociado
a la mortalidad a 2 años, con una supervivencia significativamente mayor que el grupo de pacientes con
Leucocitos al diagnóstico menor a 50 000.
Palabras clave:
Leucemia Mieloide Aguda;
Pronóstico; Supervivencia.
(fuente: DeCS BIREME)
INTRODUCTION
The acute myeloid leukemia (AML) is a type of cancer which is characterized by the infiltration of bone marrow, blood and other tissues through a clonal proliferation, abnormally differentiated and occasionally poorly differentiated from cells of the hematopoietic system1.
Considered an incurable disease for 50 years, with the current treatments there is a rate of healing from 35 to 40% in patients under 60 years old and from 5 to 15% in patients over 60 years old1.
The main factors that determine the AML prognosis can be divided in the following groups: the ones related to the patients characteristics and the ones related to the biological characteristics of the own disease. Cytogenetics and immunophenotypic data obtained by Flow Cytometry are currently the most important prognosis factors for predicting survival in patients, as the identification of different molecular alterations has made it possible to refine the prognosis by establishing risk groups in AML, while assisting during follow-up by determining the minimum residual disease (ERM)4.
In international databases, only articles are found that study the overall survival of leukemia in our country without specifying the subtype, and no survival tests are found that take clinical, cytogenetic and immunophenotypic variables.
The reason for this study will be to find the relationship between the prognosis factors in AML and survival in the adult population of Hospital Nacional Dos de Mayo, in order to use the results obtained to improve treatment decisions, using more aggressive treatments when necessary based on the prognosis study of each patient individually.
METHODS
Observational, cross-sectional, retrospective and analytical study, the data was obtained by means of a collection sheet prepared for this study of the medical records and review of the Flow Cytometry and Karyotype file of the Hematology service of all adult patients diagnosed with Acute Myeloid Leukemia, from April 2014 to December 2016 at Hospital Nacional Dos de Mayo. The final population of study were 39 patients.
The following variables were studied: age at diagnosis, sex, leukocyte count at diagnosis, type of Acute Myeloid Leukemia (De Novo or Secondary), type of Acute Myeloid Leukemia according to French-American-British Classification (FAB), cytogenetic alterations, immunophenotypic alterations, overall survival at six months after diagnosis, overall survival at one year after diagnosis, overall survival at two years after diagnosis, survival time in months.
The age at diagnosis was classified as over 60 years old and under 60 years old, the leukocyte count at diagnosis as greater than 50,000 per mm3 and less than 50,000 per mm3, the type of AML according to etiology in De Novo and Secondary, according to FAB classification in good prognosis and bad prognosis, according to cytogenetics in good prognosis and bad prognosis and according to immunophenotype in good prognosis, good and bad prognosis and bad prognosis.
STATISTICAL ANALYSIS
The collected information was ordered in a database of Microsoft Excel 2013 program, and was transferred to SPSS 24 version, software in which all statistical analyzes were performed. A descriptive analysis of variables was carried out in order to characterize the patients according to frequencies and the variables distribution to be studied.
Then analytical statistics were performed seeking to associate prognosis factors with survival; bivariate analysis was performed with determination of OR using contingency tables, 95% confidence interval, with p value (<0.05).
To determine the survival curves, the Kaplan-Meier method was performed. The comparison between the survival curves was performed using the log-rank test with statistical significance determination.
Multivariate analysis was not performed due to only finding statistical significance p (<0.05) in one of the study variables.
RESULTS
Seventy patients were registered in the database of the Hematology service with a diagnosis of Acute Myeloid Leukemia at the time of the study; of them, the data of 39 patients were analyzed because 31 patients were excluded due to having another final diagnosis or not receiving a proper follow-up by the hematology service; the basal characteristics of the population are described in Table 1
Table 1.Basal characteristics of the population.
|
Age at diagnosis: Over 60 years old: 16 (41%), under 60 years old (59%) |
|
Sex: Male: 25 (64%), Female: 14(36%) |
|
Leukocyte count at diagnosis: Greater than 50 000: 13 (13%) less than 50 000: 26 (66%) |
|
Type of AML: de Novo: 33 (84%), secondary: 6 (16%) |
|
Type of AML (FAB): Good prognosis: 18 (46%), Bad prognosis: 10 (25%), not found: 11 (28%) |
|
Cytogenetics: Good prognosis: 8 (20%), not found: 31 (80%) |
|
Immunophenotype: Good prognosis: 1 (2%), bad prognosis: 3 (8%), good t bad prognosis: 15 (38%), not found: 20 (51%) |
|
6-month survival: Yes: 25 (64%), no: 14 (36%) |
|
Survival per year: Yes: 18 (46%), no: 21 (53%) |
|
2-year Survival: Yes: 18 (46%), no: 21 (53%) |
|
Survival Time: Median: 10 months, average: 18 months |
Subsequently, contingency tables were made for the bivariate analysis with calculation of the odds ratio (OR) comparing the dependent variables with survival in the 3 times studied, 6 months, 1 year and 2 years, in order to find a positive or negative association with this of the different prognosis factors.
When performing the bivariate analysis with the variable Leukocytes at diagnosis and 6-months survival, an OR of 1.9 (95% CI: 0.489 - 7.605) was obtained, considering having a Leukocyte value at diagnosis greater than 50,000 as a risk factor for mortality, without statistical significance.
However, when analyzing the variable Leukocytes at diagnosis with survival per one year and two years after diagnosis, the same OR of 4.5 (95% HF: 1,008 - 20,507) was obtained, considering having a Leukocyte value at diagnosis greater than 50,000 as a risk factor for mortality.
The different ORs found for the study variables are summarized in Table 2.
Survival analysis was performed according to the Kaplan Meier method, using the log-rank test to compare the survival curves with the p value determination for statistical significance in the various study variables.
When performing the survival analysis of the leukocyte variable at diagnosis in the 2 prognosis groups, first with survival at 6 months, a p value = 0.3 was obtained, having no statistical significance when comparing survival among patients with leukocytes at diagnosis greater than 50,000 and less than 50,000, having a greater cumulative survival in the group of patients with leukocytes less than 50,000. (See chart 1).
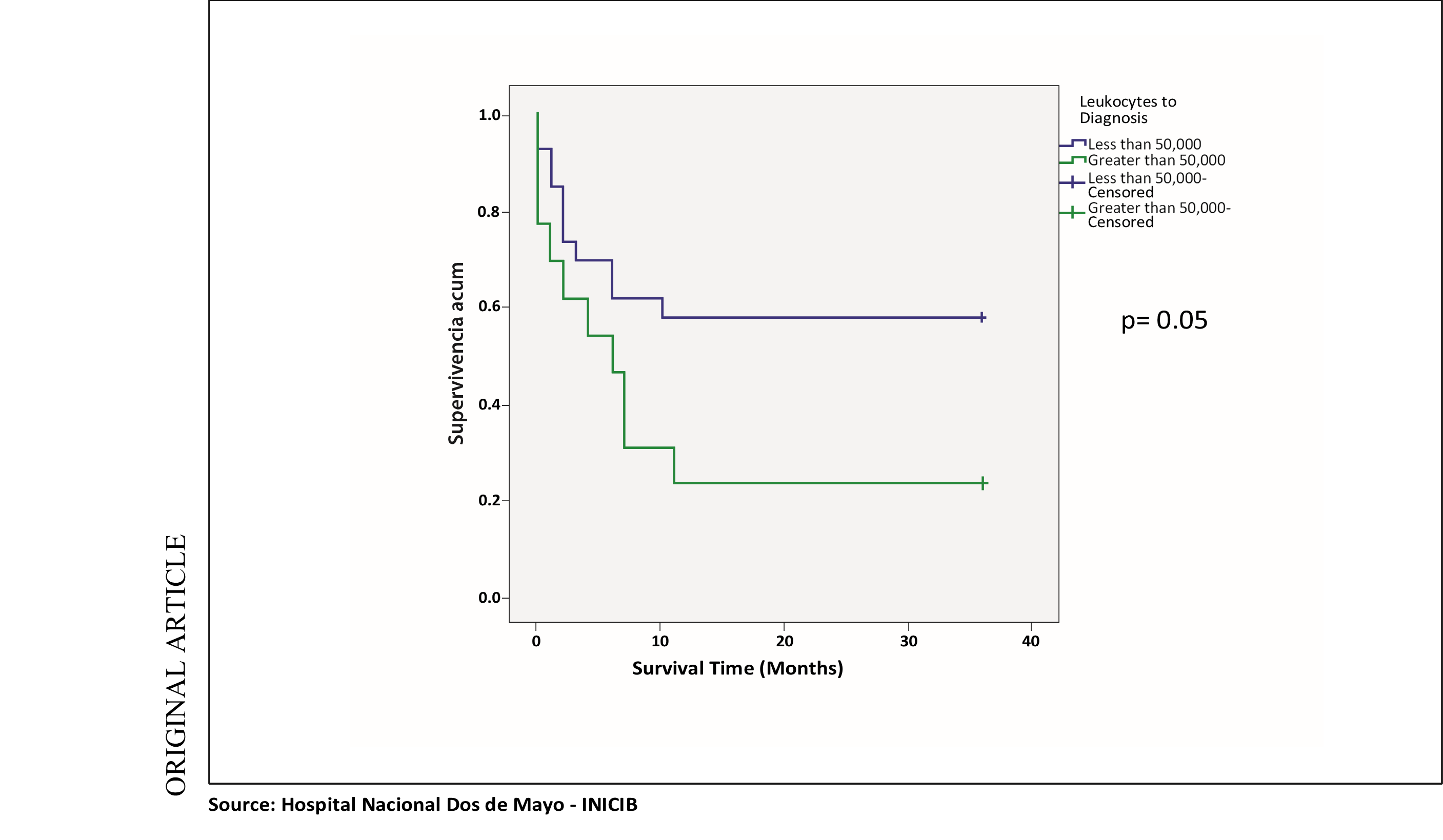
In the survival analysis of the same variable mentioned with survival at one year and two years, the same p = 0.05 value was obtained, with survival being significantly lower in the group of patients with leukocytes at diagnosis greater than 50,000 with lower cumulative survival in this mentioned group. (See chart 2).
Statistical significance was not obtained in the survival analysis of the other study variables.
Table 2.Summary of the bivaried analysis (OR found in the study variables).
|
Age at diagnosis: |
|
|
Over 60 years old Under 60 years old |
6-month survival: 0.7 (95% HF: 0.2-2.7) 2-year survival: 1.1 (95% HF: 0.3-4.2) |
|
Leukocytes to Diagnosis |
|
|
Greater than 50,000 Less than 50,000 |
6-month survival: 1.9 (95% HF: 0.4-7.6) 2-year survival: (95% HF: 1.008-20.5) |
|
Type of AML |
|
|
De Novo Secondary |
6-month survival: 0.8 (95% HF: 0.1-5.5) 2-year survival: (95% HF: 0.1-4.7) |
|
AML Type (FAB Classification) |
|
|
Good Prognosis Bad Prognosis |
6-month survival: 0.8 (95% HF: 0.1-4.5) 2-year survival: 0.6 (95% HF: 0.1-3.1) |
|
Alterations |
|
| Immunophenotypic: Good and bad Prognosis Bad Prognosis |
2-year Survival: 0.3 (95% HF: 0.02-5.21) |
Chart 1. Survival curve at 6 months: Leukocytes to diagnosis and survival at 6 months.
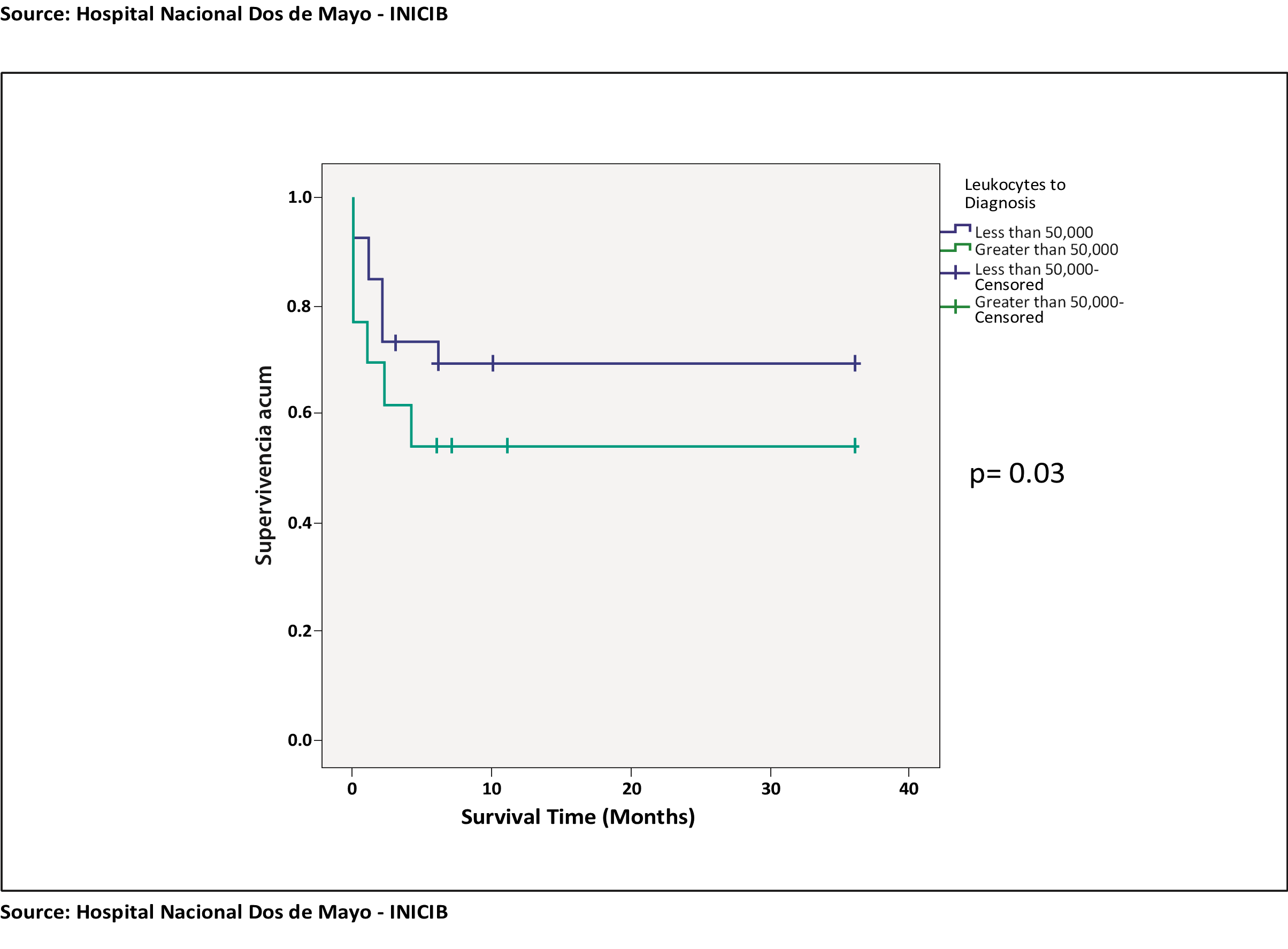
Chart 2. Survival curve at 2 years: Leukocytes to diagnosis and survival at 1 and 2 years
DISCUSSION
Acute myeloid leukemia is a public health problem that, as already mentioned, has a high burden of disease and also generates high costs for its treatment, which is why knowing the prognosis factors, clinical, immunophenotypic, and cytogenetic has vital importance in order to give an adequate individualized treatment to each patient3.
In our study, we found the age at diagnosis divided into 2 prognosis groups: patients under 60 years old and over 60 years old. When determining the risk with the OR, it was found that being older than 60 years old would constitute a mortality factor, without statistical significance in our population and the survival analysis, it was also found that patients under 60 years old had a higher mortality, without statistical significance at 6 months, 1 year and 2 years with a p value greater than 0.05. When compared with the study by Alberto D. Giménez Conca8, et al, in their study "Variables condicionantes al tratamiento en pacientes añosos con Leucemia Mieloide Aguda, Experiencia Institucional" in 2016, a retrospective cohort that included 133 patients, demonstrated by the analysis of the Kaplan Meier curves, a median survival of 1.7 months in those older than 70 years old, and 4.7 months for those younger than 70 years old, p <0.001, being 70 years old had a Hazard Ratio of 2.09 of risk of mortality in this study population8. Subsequently, when compared with the study by Betul Oran11, et al, "Survival for older patients with acute myeloid leukemia: a population based Study" in 2012, it was a retrospective cohort study that included 5480 patients with diagnosis of AML, with a median age of 78 years old, range from 65 to 93 years old, survival was compared between the group that received chemotherapy treatment vs the group that did not receive it, finding that the median survival was two months in the group that did not receive chemotherapy compared to 6-months group that received it (p <0.01), with the best survival in patients aged 65-69 years (10 months vs 4 months) (p <0.01) and 70-74 years (8 months vs 3 months) (p <0.01). 46 patients who received hematopoietic stem cell transplantation (0.8%) had a median survival of 22 months. Better survival is always observed in younger patients with proper statistical significance.
At the national level, the study by Pedro Eduardo Lovato18 “Leucemia Mieloide aguda en adultos: Estudio comparativo sobre tratamiento y pronóstico por grupos etarios” in 2015, this descriptive study made in 208 patients, 153 young adults and 55 older adults, had as objective to compare the evolution of patients younger than 60 years olds and older patients that received chemotherapy, made at Hospital Edgardo Rebagliati Martins between January, 1995 and December, 2008. It was found that associated mortality to induction treatment and rate of complete remission showed statistically significant differences between the young adults group and the older adults one (p=0.048 and p = 0,02 respectively), favouring the young adults groups with less mortality. Being 17.85% at 5 years the total survival in the young adults group, with a survival median of 11.03 months, while the total survival in adults older than 5 years was 12.14% with a survival median in 6 months18.
Regarding leukocytes at diagnosis, it was divided into two prognosis groups: Greater than 50,000 and less than 50,000, we found in our study, in the determination of risk with the OR, that having leukocytes greater than 50,000 is associated as a risk factor for mortality at one year and two years with statistical significance and in the survival curves, it was also found that survival at one year and two years was significantly lower in the group of patients with leukocytes at diagnosis greater than 50,000 with a p value lower than 0.05. When contrasted with the study by Alberto D. Giménez Conca, et al, a retrospective cohort study that included 133 patients, the leukocyte count greater than or equal to 30,000 showed a HR of 1.63 as a factor of bad survival prognosis.
In the variable type De Novo or Secondary AML, we found that in risk determination with the OR that having De Novo leukemia, not associated with a record of previous chemotherapy or myelodysplastic syndrome, would be a protective factor for mortality, without statistical significance, likewise in the survival curves, a greater survival was found in patients with Secondary Leukemia, however this finding was not statistically significant.
As mentioned in the study by Perea Durán G, patients who have secondary acute leukemia to previous radiochemotherapy treatment or after myelodysplasia frequently present as resistant leukemia and present prolonged periods of post-treatment cytopenia, which will favour post infectious complications4. In addition, secondary AML is associated with bad prognosis cytogenetic alterations, particularly chromosome abnormalities 5 and 7 in patients previously treated with alkylating agents and deletions of the long arm of chromosome 11 in patients treated with Topoisomerase II inhibitors.
In our study we found when dividing Leukemia according to its classification (French - American - British) (FAB), into groups with good prognosis and bad prognosis, when performing the risk analysis that having the subtypes of AML considered to be of bad prognosis would be a protective factor for mortality, however without statistical significance, and in the survival curves, we found greater survival in patients with bad prognosis subtypes, but without statistical significance. As already described in the study by Perea Durán G, the morphological subtypes M6 and M7 are considered to have a worse prognosis due to their strong association with unfavorable cytogenetic abnormalities4.
Currently, the identification of the immunophenotypic alterations found in flow cytometry, the cytogenetic alterations studied in the karyotype and also the determination of molecular biology, a non-studied-variable in this work, are the most important factors when determining the prognosis and individualizing the treatment of each patient diagnosed with Acute Myeloid Leukemia.
This study had many limitations when studying these variables, since in many of the medical records reviewed, it happened that when reviewing them and even when reviewing the Flow cytometry and Karyotype file of the Hematology service as described, we could not count with the entire study. This was also influenced by the fact that many of the patients died at the onset of their disease, either with intracranial hemorrhage or upper gastrointestinal hemorrhage as the most frequent causes, and with a suggestive hemogram of Acute Myeloid Leukemia. In these cases, there was not the time necessary to carry out the appropriated examinations that in the case of Hospital Nacional Dos de Mayo have been ordered to be carried out at Instituto Nacional de Enfermedades Neoplásicas for several years.
According to prognosis groups based on the immunophenotypic alterations of the flow cytometry, when performing the risk analysis with the OR in patients with whom the study was included, it was obtained that having only alterations considered to be of bad prognosis, comparing the group that had a good and bad prognosis, as a protective factor for mortality, had no statistical significance. And in the survival analysis, we obtained that there was no statistical significance when comparing survival between the group with alterations with a good prognosis, a bad prognosis, and those who had both types of alterations, with higher mortality in those patients with both types of alterations.
As mentioned, we only counted on the cytogenetic study of 8 patients, reason why the corresponding analysis of survival could not be made.
Despite the results obtained, the literature contrasts the importance of these tests in the prognosis of these patients, as in the study by Juan Felipe Combariza Vallejo5 “Cohorte de supervivencia en pacientes menores de 60 años con leucemia mieloide aguda de acuerdo a la citogenética y el tratamiento de consolidación” in 2015, they evaluated survival, prognostic factors and their association with the initial karyotype in a retrospective cohort way in a population of 66 patients with AML younger than 60 years old. A overall survival range at 2 years of 90% for low risk groups, 61% for intermediate risk and 30% for high risk (p = 0.016) was found. With a complete response to induction therapy in patients with low risk of 90.9%, intermediate risk 77.8% and high risk of 60%5. Harry Dang12, et al, in their study "Prognostic value of immunophenotyping and gene mutations in elderly patients with acute myeloid leukemia with normal karyotype" showed that the expression of CD56 was an independent factor of bad prognosis, with a lower percentage of complete remission (p = 0.021) and event-free survival (p = 0.003). In addition, the expression of CD34 was correlated with lower overall survival (13 months vs. 22.6 months, p = 0.015)12.
Regarding the molecular biology studies that at the moment are starting to be carried out routinely in all patients at Hospital Nacional Dos de Mayo, they take vital importance for an individualized management as demonstrated by Pierre Hirsch14, et al. in their study “Acute myeloid leukemia in patients older than 75: Prognostic impact of FLT3-ITD and NPM1 mutations” in a population of 79 patients older than 75 years old. NPM1 mutations were associated with a higher rate of complete remission (p = 0.12), while FLT3-ITD mutation was associated with a worse survival (p = 0.049)14 .
CONCLUSION
Statistical significance was found in our study population when evaluating the clinical variables. It was obtained with the variable Leukocytes at diagnosis greater than 50,000, finding it as a factor associated with 2-year mortality, with a significantly higher survival than the group of patients with Leukocytes at diagnosis less than 50,000.
In the FAB classification, we did not obtain statistical significance in our study population regarding survival between good and bad prognosis groups.
We were unable to perform the survival analysis of the cytogenetic alterations variable due to the aforementioned limitations. Regarding immunophenotypic alterations, we did not obtain statistical significance in our study population regarding survival between good and bad prognosis groups.
Recommendations
It is recommended to carry out larger studies, covering a longer period of time at Hospital Nacional Dos de Mayo, in order to obtain statistical significance.
In the coming years, due to the routine performance of flow cytometry, karyotype and molecular biology studies in all patients with a Acute Myeloid Leukemia diagnosis at Hospital Nacional Dos de Mayo, it will be possible to carry out more extensive studies to obtain all studies in these patients.
It is recommended to carry out other works involving other hospitals in Lima and province, of EsSalud and Minsa, in order to obtain general data on the national population with a diagnosis of AML.
Authorship contributions: The authors participated in the production, collection of information, writing and final approval of the original article.
Financing: Self-financed.
Conflict of interest: The authors declare there is no conflict of interest in this article publication.
Received: October 30, 2018
Approved: January 02, 2019
Correspondence: Nicolás J. Higueras Bromley
Address: CL Sor Tita 306 Int. 501 Urb. El Rosal, Santiago de Surco- Lima.
Telephone: +51 7665953
Email: nhb1994@gmail.com
BIBLIOGRAPHICAL REFERENCES
-
1. Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015; 373(12):1136-52.
2. Leucemia mieloide aguda en adultos [Internet]. National Cancer Institute. [Citado 16 de julio de 2018]. Disponible en:https://www.cancer.gov/espanol/tipos/leucemia/pro/tratamiento-lma-adultos-pdq
3. Willy César Ramos Muñoz. La carga de Leucemias en Perú, Boletín Epidemiológico. Boletín Epidemiológico, Lima. 2014; 23:630-1.
4. Perea Durán G. Factores pronósticos en leucemia mieloide aguda: utilidad de los estudios inmunofenotípicos y moleculares [Internet]. Universitat Autònoma de Barcelona,; 2011 [citado 16 de julio de 2018]. Disponible en: https://ddd.uab.cat/record/127367
5. Combariza-Vallejo JF. Cohorte de supervivencia en pacientes menores de 60 años con leucemia mieloide aguda de acuerdo con la citogenética y el tratamiento de consolidación. Iatreia [Internet]. 2015 [citado 16 de julio de 2018]; 28(4). Disponible en: http://www.redalyc.org/resumen.oa?id=180541348003
6. Yébenes-Ramírez M, Serrano J, Martínez-Losada C, Sánchez-García J. Factores pronósticos clínico-biológicos en pacientes con leucemia aguda mieloblástica en recaída. Med Clínica. 2016; 147(5):185-91.
7. González B, Bueno D, Rubio PM, San Román S, Plaza D, Sastre A, et al. Aspectos inmunológicos de la leucemia mieloblástica aguda. An Pediatría. 2016; 84(4):195-202.
8. Conca G, D A, Arbelbide JA, Schutz N, Otero V, Fantl D, et al. Variables condicionantes del tratamiento en pacientes añosos con leucemia mieloide aguda: Experiencia institucional. Med B Aires. 2016; 76(2):81-8.
9. Kandeel EZ, El Sayed G, Elsharkawy N, Eldin DN, Nassar HR, Ibrahiem D, et al. Impact of FLT3 Receptor (CD135) Detection by Flow Cytometry on Clinical Outcome of Adult Acute Myeloid Leukemia Patients. Clin Lymphoma Myeloma Leuk. 2018.
10. Manola KN, Panitsas F, Polychronopoulou S, Daraki A, Karakosta M, Stavropoulou C, et al. Cytogenetic abnormalities and monosomal karyotypes in children and adolescents with acute myeloid leukemia: correlations with clinical characteristics and outcome. Cancer Genet. 2013; 206(3):63-72.
11. Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012; 97(12):1916-24.
12. Dang H, Jiang A, Kamel-Reid S, Brandwein J, Chang H. Prognostic value of immunophenotyping and gene mutations in elderly patients with acute myeloid leukemia with normal karyotype. Hum Pathol. 2013; 44(1):55-61.
13. Juncà J, Garcia-Caro M, Granada I, Rodríguez-Hernández I, Torrent A, Morgades M, et al. Correlation of CD11b and CD56 expression in adult acute myeloid leukemia with cytogenetic risk groups and prognosis. Ann Hematol. 2014; 93(9):1483-9.
14. Hirsch P, Qassa G, Marzac C, Tang R, Perrot J-Y, Isnard F, et al. Acute myeloid leukemia in patients older than 75: prognostic impact of FLT3ITD and NPM1 mutations. Leuk Lymphoma. 2015; 56(1):147-50.
15. Stölzel F, Mohr B, Kramer M, Oelschlägel U, Bochtler T, Berdel WE, et al. Karyotype complexity and prognosis in acute myeloid leukemia. Blood Cancer J. 2016; 6:e386.
16. Damiani D, Tiribelli M, Raspadori D, Sirianni S, Meneghel A, Cavalllin M, et al. Clinical impact of CD200 expression in patients with acute myeloid leukemia and correlation with other molecular prognostic factors. Oncotarget. 2015; 6(30):30212-21.
17. Tello-Vera S, Colchado-Aguilar J, Carpio-Vásquez W, RodríguezGueorguiev N, Díaz-Vélez C. Supervivencia de pacientes con leucemias agudas en dos hospitales de la seguridad social del Perú. Rev Venez Oncol. 2018; 30(1):2-9.
18. Lovato PE. Leucemia mieloide aguda en adultos: Estudio comparativo sobre tratamiento y pronóstico por grupos etarios. Rev Medica Hered. 2015; 26(3):160-6.
19. Vasquez P, Patricia K. Caracterización Inmunofenotípica Y Citogenética De Pacientes Con Leucemia Mieloide Aguda Instituto Nacional De Enfermedades Neoplásicas. Univ Nac Federico Villarreal [Internet]. 2018 [citado 16 de julio de 2018]; Disponible en: http://repositorio.unfv.edu.pe/handle/UNFV/1945



