CLINICAL CASE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2020 - Universidad Ricardo PalmaDOI 10.25176/RFMH.v20i4.2927
ACUTE APPENDICITIS IN INTESTINAL MALROTATION. CASE REPORT
APENDICITIS AGUDA EN MALROTACIÓN INTESTINAL. REPORTE DE CASO
Febres-Ramos R1, a, Nelson F. Diaz-Reyes1, 2, b
1Unidad de Docencia e Investigación - Clínica Adventista Good Hope.
2Universidad Peruana Unión.
aSurgeon
bHead of Department, Teacher
Intestinal malrotation is a congenital abnormality of the intestine’s embryological rotation, which develops: Ladd's bands or a narrow mesenteric base, predisposing to gastrointestinal obstruction. It is estimated that more than 90% of patients will present in the first 12 months of life. In newborns, malrotation presents with acute obstruction or volvulus; however, it may go unnoticed in some patients.
The treatment of choice for intestinal malrotation is elective surgery, with the Ladd procedure, by laparoscopy or open surgery, in which duodenal fibers are cauterized. As for the management of asymptomatic patients, prophylactic surgical correction is recommended in children’s case and observation in adults, since the acute presentation in these is infrequent. In an acute presentation in adults, emergency laparotomy is required to determine the cause and avoid the intestine’s necrosis. A case of the acute surgical abdomen due to acute appendicitis is presented in a 27-year-old female patient with intestinal malrotation, with initial clinical presentation of diffuse abdominal pain, lumbar pain, positive lumbar percussion fist, which finally reached an adequate resolution and a good prognosis.
Key words: Acute Appendicitis, Intestinal malrotation (Source: MeSH NLM).
RESUMEN
La malrotación intestinal es una anormalidad congénita de la rotación embriológica del intestino, que desarrolla: bandas de Ladd o una base mesentérica estrecha, que predisponen a obstrucción gastrointestinal. Se estima que más del 90% de pacientes se presentará en los primeros 12 meses de vida. En los recién nacidos, la malrotación se presenta con obstrucción aguda o vólvulo, sin embargo, en algunos pacientes pueden pasar desapercibidos.
El tratamiento de elección para la malrotación intestinal es la intervención quirúrgica electiva, con el procedimiento de Ladd, por laparoscopía o cirugía abierta, en la que se cauterizan fibras duodenales. En cuanto al manejo de pacientes asintomáticos se recomienda la corrección quirúrgica profiláctica en el caso de niños, y observación en adultos ya que la presentación aguda en éstos es muy rara. En el caso de presentación aguda en adultos se requiere laparotomía de emergencia para determinar la causa y evitar la necrosis del intestino. Se presenta un caso de abdomen agudo quirúrgico por apendicitis aguda en una paciente mujer de 27 años con malrotación intestinal, con presentación clínica inicial de dolor abdominal difuso, dolor lumbar, puño percusión lumbar positiva, que finalmente llegó a una resolución adecuada y un buen pronóstico.
Palabras Clave: Apendicitis Aguda, Malrotación intestinal (fuente: DeCS BIREME).
Intestinal malrotation (MRI) is a congenital abnormality of the intestine’s embryological rotation, which develops: Ladd's bands or a narrow mesenteric base, which predispose to gastrointestinal obstruction(1). It is estimated that more than 90% of patients will present in the first 12 months of life(2). In newborns, MRI shows with acute obstruction or volvulus(3); however, it may go unnoticed(1).
Rotation and fixation abnormalities of the gastrointestinal tract are frequently associated with abdominal wall abnormalities and diaphragmatic hernia. Filston and Kirks report an association of up to 62% with lesions such as atresias, stenosis of the upper gastrointestinal tract, intussusception, and Hirschsprung's disease(4, 5). It encompasses a wide variety of bowel rotation and fixation abnormalities; the same alteration can remain asymptomatic throughout life or produce an acute abdomen that ends the life of the patient if it is not diagnosed and treated appropriately(5).
The incidence of MRI is difficult to determine, being reported in a varied way from approximately 1/500 live births(4), to 2.86 x 100,000 live births and fetal deaths according to an epidemiological study in Hawaii(6). However, with imaging, incidental recognition of MRI is more likely(6, 7).
The clinical presentation in adults, usually manifests insidiously during the postprandial period with intermittent vomiting, abdominal pain, food intolerance, peritonitis, and can present as acute symptoms suggestive of intestinal obstruction(4). MRI should be considered in persistent symptoms of recurrent abdominal pain or even acute abdominal pain, which is usually very difficult to diagnose. As it is of very low suspicion, especially in the adult population, which mostly begins as asymptomatic, MRI pathology is detected in scenarios of incidental finding of images or often associated with other anatomical alterations(4, 5). MRI diagnosis should be suspected in adults with clinical or imaging findings(6). The barium radiograph with high intestinal transit is the Gold Standard for the diagnosis of an MRI(7, 8, 9). If there is an acute clinical presentation of MRI, the finding is usually made intraoperatively(10).
The treatment of choice for MRI is elective surgery, with the Ladd procedure, by laparoscopy or open surgery, in which duodenal fibers are cauterized(4,10,11).
Regarding asymptomatic patients’ management, prophylactic surgical correction is recommended in children’s case and observation in adults, since the acute presentation is infrequent(4). In an acute presentation in adults, emergency laparotomy is required to determine the cause and avoid necrosis of the intestine(11).
On the other hand, acute appendicitis (AA) is the most common acute surgical pathology in adults. Typical symptoms include periumbilical pain that migrates to the right iliac fossa, anorexia, fever, and evidence of peritoneal irritation(5). Due to the variety of appendicular positions, one-third of AA patients have pain located outside the right lower quadrant. In both children and adults, the left cecal appendix can present in situs inversus totalis or in MRI(5).
The cecal appendix’s approximate size is 9–10 cm in adults, which implants the cecum’s lower border, about 3 cm below the ileocecal valve(7, 12). While the relationship between the base of the appendix and the cecum is constant, the distal end is mobile and capable of changing position, retrocecal, retroileal, preileal, subcecal, or pelvic(7,8).
In a study carried out in eight patients with computed tomography (CT) findings of complicated AA in adults associated with MRI, it was determined that all patients had an average of 1 to 5 days of abdominal pain. Diffuse abdominal pain occurred in two, left abdominal pain in two, lower abdominal pain in two, and right lower quadrant pain in the last two patients.(11)
DESCRIPTION OF THE CLINICAL CASE
The patient is a 27-year-old female from Canada who attended the health facility in December 2018, with a sickness time of 06 hours, where she reported that upon waking up. She presented diffuse abdominal pain of moderate-intensity (5/10). The patient eats breakfast, but persists pain and is associated two hours after having breakfast, diarrhea episodes, nausea, and vomiting; the pain radiates to the right shoulder. She takes paracetamol, which slightly decreases the pain, but then intensifies. The discomfort continues with colicky abdominal pain predominantly in the right upper quadrant and low back pain of moderate-intensity (6/10) is added at the L1 level, which is why she goes to the emergency room .
To evaluation; hemodynamically stable, in apparent good general, nutritional and hydration status. Symmetrical flat abdomen, no scars or masses, decreased air-fluid sounds (+ / ++) soft, depressible, painful on deep palpation in the right upper quadrant, with an impression of peritoneal signs, McBurney and positive Blumberg; slight muscular resistance in the right flank. Genito-urinary with right PPL (+). Rest of the non-contributory exam.
Surgery was consulted, after taking blood and laboratory tests, abdominal ultrasound (Figure 1), plain abdominal radiography in a standing position (Figure 2)

Figure 2. Plain standing abdominal X-ray. Shadow of the intra-abdominal organs of normal shape and position.
Laboratory tests: blood group A negative. Urine Exam: Not pathological. Serologic: HCG - beta subunit (quantitative), blood culture, urine culture, Hepatitis B antigen, HIV I - II (ELISA), and VDRL negative.
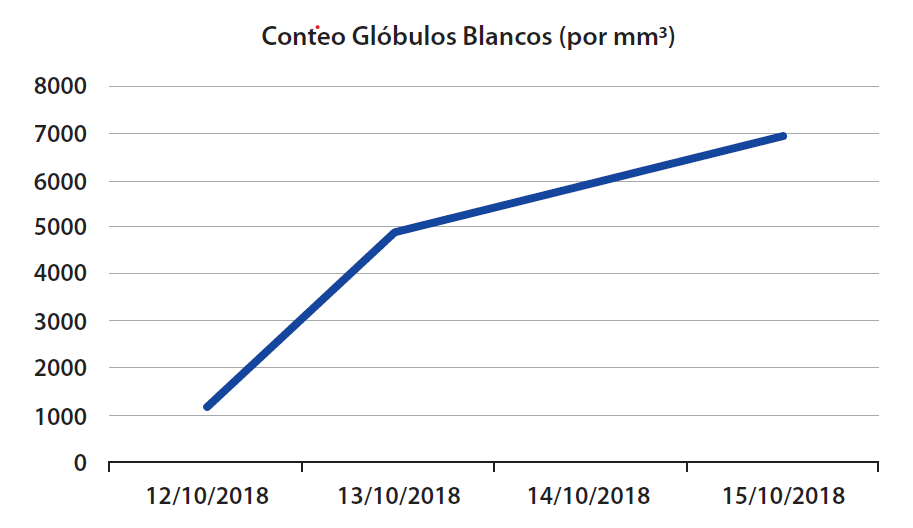
The surgeon decides to observe for 12 hours with intravenous hydration with isotonic crystalloids. Both the leukocyte count and the quantitative C-reactive protein value increased slightly. compared to the day of admission. (Table 1)
Table 1. Laboratory Data
| Variable | Reference Values | 12/10/2018 | 13/10/2018 |
| Hematocrit (%) | 36–46 | 44.1 | 36.8 |
| Hemoglobin (g/dl) | 12–16 | 15.1 | 12.2 |
| White blood cell count (per mm3) | 4500–11,000 | 1210 | 4960 |
| Differential count (%) | |||
| Neutrophils | 40–70 | 87 | 70.1 |
| Lymphocytes | 22–44 | 9 | 19.2 |
| Monocytes | 4–11 | 4 | 9.9 |
| Eosinophils | 0–8 | 0 | 0 |
| Basophils | 0–3 | 0 | 0 |
| Stuck | 0-4 | 0 | 0 |
| Platelets (per mm3) | 150,000–400,000 | 250,000 | 209,000 |
| Red blood cell count (per mm3) | 4,000,000–5,200,000 | 4,570,000 | 3,730,000 |
| corpuscular volume (fl) | 80–100 | 96.50 | 98.70 |
| Creatinine (g/dL) | 0.50-0.90 | 0.82 | |
| Urea (g/dL) | 10-50 | 17.0 | |
| Oxaloacetic transaminase (U/liter) | 7–33 | 15.5 | |
| Pyruvic transaminase (U/liter) | 9–32 | 20.5 | |
| Amylase (g/dL) | 28-100 | 51 | |
| Electrolytes | |||
| Sodium | 135-147 | 139.9 | |
| Potassium | 3.5-5.0 | 3.62 | |
| Chlorine | 95-111 | 102.7 | |
| C-reactive protein | 0-0.5 | 4.240 | 10.220 |
Given the persistence of abdominal pain predominantly in the right iliac fossa of intensity (8/10), positive Mc Burney's sign, an abdominopelvic tomography with contrast was performed. (Figure 3 y 4).

Figure 3. 24 hours after admission. Multislice spiral tomography of the abdomen and pelvis, presence of air in the intestines in the right iliac fossa and pancreas.

Figure 4. 24 hours after admission. Multislice spiral tomography of the abdomen and pelvis (10/13/2018): Presence of loops with edematous walls and air in the intestines.
Subsequently, with the abdomen’s multislice spiral tomography pelvis’s help, imaging studies that were not conclusive and given the persistence of the symptoms of intensity abdominal pain in the right iliac fossa (9/10). Positive McBurney sign; acute abdominal pain syndrome problems were raised, with a possible point of appendicular origin.
Due to clinical suspicion, like all appendicular symptoms, the surgeon decides to perform exploratory laparoscopic surgery. Antibiotic prophylaxis with intravenous ceftriaxone and metronidazole was performed before the procedure.
Surgical technique: general anesthesia, pneumoperitoneum with Veress at 14 mmHg, umbilical T1, suprapubic T2 and left flank T3. During the intraoperative period, the following were observed: congenital MRI, which determines the medial position of the cecum, absence of the ascending colon, retrocecal appendix with congestive phase. Given the MRI diagnosis due to an incidental finding in the intraoperative period, the clinical outcome or the treatment indicated for AA is not modified, so we proceed to perform laparoscopic appendectomy under the usual techniques. After the surgical intervention, he went to recovery, with hemodynamic stability.
In the postoperative period, the patient is afebrile, with mild pain in the operative area, predominantly umbilical, eliminates flatus, received intravenous analgesic therapy with ketoprofen, tramadol and gastric protection. There was no evidence of intestinal obstruction on the second postoperative day, tolerating the oral route, with a favorable clinical evolution, which is why discharge was decided on the third day.
DISCUSSION
The patient was admitted for diffuse abdominal pain and lumbar pain, suggestive of an upper urinary infection, ruled out with an RCT and negative urine culture. However, due to the suspicion of a clinical picture of AA, the clinical presentation was not entirely clear. We had to rely on the study of images, which were not conclusive either, and finally the intraoperative diagnosis had to be reached. This is important to emphasize due to the problematic diagnostic and treatment approach in patients with MRI associated with appendicitis(5). There is evidence that the presentation of appendicitis is altered when it manifests in individuals with abnormal location of the abdominal viscera(5, 13). On the other hand, there are MRI or abnormal descents of the cecum that are associated with abnormal locations of the appendix that influences the clinical presentation of appendicitis, as was the case in this case(8).
So far and when in doubt, it should be remembered that the Gold Standard for the diagnosis of AA is the multislice abdominal helical tomography with contrast(8, 14), having a sensitivity of 100%, specificity of 81.8%(6, 8).
Similarly, our environment’s facilities allowed an adequate, cost-effective study such as abdominal tomography, as reported by Ben Ely et al(11) for the diagnosis of MRI, which unfortunately was not conclusive (Figure 3 and 4) and the findings had to be corroborated intraoperatively.
Although the leukocyte count and the C-reactive protein value increased slightly compared to the day of admission, this defined the role that peritoneal macrophages play in response to stress due to intra-abdominal surgery(8, 11), where there is a decrease in phagocytosis and increased cytokine production, immunosuppression at the local level. However, it has been shown that their exposure to ambient air causes a neuroendocrine response to stress that is much greater and more damaging than that produced during laparoscopic surgery(9, 11, 12). Therefore, we can explain this phenomenon as a consequence of the appendicular picture and an idiosyncratic response of the body to surgical intervention(11, 15).
Lithiasic uropathies and urinary tract infections are common pathologies in young female patients(10, 15), especially with the presentation of colicky abdominal pain that does not ease quickly with analgesic alone(12, 14). They were considered essential differential diagnoses due to low back pain and positive lumbar percussion fist; however, the physical examination and negative auxiliary examinations ruled them out.
It is stipulated that there is a relationship between the clinical and surgical manifestations of MRI and its irregular location(2, 11). This pathology usually has a favorable prognosis, being the expressions with hemodynamic compromise necessary for surgical management or intensive medicine due to high risks of organ failure and concomitant infections, being the most deadly(4, 10, 11). However, the patient’s findings showed us that the typical manifes
Finally, there are few cases reported worldwide of congenital MRI, being of these few those that lead to the presentation of a surgical manifestation with a clear clinical picture(4), 14. Usually, these abnormal positions in healthy individuals are underdiagnosed, and if they are, they are classified as incidental(9
CONCLUSIONS
Taking as an essential precedent the diagnostic difficulty when evaluating a patient with MRI pathology associated with AA, it is recommended that the existence of abdominal pain in the right upper quadrant should be taken into account in order to rule out acute surgical pathology by MRI. Although its incidence worldwide is low, the initial diagnosis and timely management of the patient can reduce complications.
Author’s contributions: The authors participated in the genesis of the idea, the design, the collection of information, the analysis of the results, and the preparation of the manuscript.
Funding: Self-financed.
Conflicts of interest: The authors declare that they have no conflicts of interest due to what is mentioned in this communication.
Received: May 26, 2020
Approved: June 10, 2020
Correspondence: Richard Jeremy Febres Ramos
Address: Pje. Alejandro O´ Deustua N° 138
Telephone: 990009956
E-mail: richardfr.94@gmail.com
BIBLIOGRAPHIC REFERENCES