CORRECTED ARTICLE
ORIGINAL PAPER
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2020 - Universidad Ricardo PalmaDOI 10.25176/RFMH.v20i4.3057
ASSESSMENT OF QUESTIONNAIRE MEASURING CLIMATE AND MENOPAUSE AT THE FIRST LEVEL OF CARE
INSTRUMENTO PARA LA EVALUACIÓN DEL CLIMATERIO Y LA MENOPAUSIA EN EL PRIMER NIVEL DE ATENCIÓN
Juan P. Matzumura-Kasano1,a, Hugo F. Gutiérrez-Crespo1,b, José Isaac Wong Mac2,c, Isabel J. Alamo-Palomino3,d
1Universidad Nacional Mayor de San Marcos, Lima-Perú.
2Policlínico Peruano Japonés, Lima-Perú.
3Hospital EsSalud Edgardo Rebagliati Martins, Lima-Perú.
aObstetrician-Gynecologist, Vice-dean for postgraduate studies and research
bMaster in Teaching and Health Research
cObstetrician-Gynecologist
dMedical Surgeon Specialist in Health Management
Introduction:The population of middle-aged women will increase in the coming years and will require greater attention to alleviate symptoms of care related to climacteric. Objective: To validate an instrument for the evaluation of climacteric and menopause in the first level of care. Methods: Cross-sectional prospective instrument validation design. Women who were treated in health establishments of the first level of care participated. The research was carried out in three phases, initial review (literature review, translation), trial (content validity, pilot test), and psychometric properties (internal consistency analysis, exploratory factor analysis). For data analysis, the SPSS version 20 program was used. Results: 136 patients participated with a mean age of 48.6 ± 5.3 years and a mean body mass index of 27.8 ± 4.4. In the first phase, the most relevant symptoms were identified, menstrual irregularities, hot flashes, vaginal problems, urinary incontinence, quality of sleep, and mood. The translation was carried out with the participation of four professionals. The second phase, of content validity, was through expert judgment, obtaining a concordance of 0.77 and initial reliability of 0.79. In the third phase, the internal consistency was 0.69 and 0.70 for exploratory factor analysis. The instrument contains six questions, obtaining ≥ 4 affirmative answers justify specialized management. Conclusion: The instrument is brief, has adequate content validity and internal consistency, useful for managing climacteric at the first level of care.
Key words: Menopause, questionnaire, primary health care (Source: MeSH NLM).
RESUMEN
Introducción: La población de mujeres de mediana edad se incrementará en los próximos años y requerirá una mayor atención para aliviar los síntomas relacionados a climaterio en la consulta médica. Objetivo: Validar un instrumento para la evaluación del climaterio y la menopausia en el primer nivel de atención. Métodos: Diseño de validación de instrumento, prospectivo y de corte transversal. Participaron mujeres que fueron atendidas en establecimientos de salud del primer nivel de atención. La investigación se realizó en tres fases, revisión inicial (revisión de literatura, traducción), ensayo (validez de contenido, prueba piloto) y propiedades psicométricas (análisis de consistencia interna, análisis factorial exploratorio). Para el análisis de datos se utilizó el programa SPSS versión 20. Resultados: Participaron 136 pacientes con una edad promedio de 48,6 años ± 5,3 y un promedio de índice de masa corporal 27,8 ± 4,4. En la primera fase se identificaron los síntomas más relevantes, irregularidades menstruales, sofocos, problemas vaginales, incontinencia urinaria, calidad de sueño y estado de ánimo. Se procedió a la traducción con la participación de cuatro profesionales. La segunda fase, de validez del contenido, fue mediante juicio de expertos, obteniendo una concordancia de 0,77 y una confiabilidad inicial 0,79. En la tercera fase, la consistencia interna fue 0,69 y 0,70 para análisis factorial exploratorio. El instrumento contiene seis preguntas, la obtención de ≥ 4 respuestas afirmativas justifican un manejo especializado. Conclusión: El instrumento es breve, tiene una adecuada validez de contenido y consistencia interna, útil para el manejo del climaterio en el primer nivel de atención.
Palabras Clave: Menopausia, Cuestionario, Atención primaria de salud (fuente: DeCS BIREME).
At present, life expectancy has increased throughout the world until the eighth decade; particularly in developed countries(1) and in some Latin America(2). By 2025, it is estimated that the number of postmenopausal women will increase to 1.1 billion worldwide and, as the world population rises, a greater proportion of this population will be made up of people over 50 years of age(3). Various epidemiological studies carried out in menopausal women have provided consistent and reliable information on the incidence, prevalence, and severity of various symptoms related to menopause(4).
One of the ovarian functions is the production of hormones (estrogens and progesterone). When the production of the follicles is depleted, it manifests itself through hormonal decline, producing menopause. The average age of menopause varies between 48 to 52 years, but with a trend towards an increasing age(5,6).
Almost three-quarters of women report symptoms considered problematic and they are the main reason for women to seek care related to physical and psychological symptoms. Vasomotor symptoms are considered the most commonly reported and can cause sleep disturbances for many months(7,8). Mood alterations and irritability are also described, while symptoms related to sexual function, which include vaginal dryness, dyspareunia, and decreased libido, affect the quality of life of women(9). On the other hand, the loss of sex hormones during aging is related to the increase in long-term risks such as decreased bone density, metabolic disease, cardiovascular disease, Alzheimer's disease, among other diseases of the high cost to societies(10).
It is important to note that the care of women over 50 years of age is a key issue for specialist doctors and general practitioners, aiming at the care and promotion of health for middle-aged women, as well as their empowerment to make positive decisions on their post-reproductive health and well-being and thus be able to access a personalized care plan, considering their short, medium and long-term goals in a context that improves their quality of life(3,11). Likewise, this population will continue to increase and it is expected that this increase will be associated with a greater number of care or referrals to attend to short-term and long-term symptoms.
Menopausal hormone therapy remains the most effective treatment for relieving vasomotor symptoms and genitourinary syndrome. The new recommendations on its use state that it is safer in women under 60 years of age, since the symptoms can persist until after 65 years. For this reason, its use is recommended individually, taking into account a risk-benefit evaluation of each patient(12). Therefore, it is required that specialists and general physicians have the skills to provide management of perimenopause, menopause, and climacteric. Also, they must be prepared to identify problems related to climacteric and manage them and/or refer the patient for specialized management(13).
The increasing prevalence of chronic diseases will make primary care needs more complex(14). It is important to note that in some Latin American countries the provision of specialized health services remains limited and scarce.
Currently, there is a need for the health system to evolve to face new challenges in light of the aging of a population with increasingly complex needs; that is, providing individualized care for each menopausal woman, taking into account the window of opportunity and other aspects for the prevention of long-term diseases and thereby promoting an improvement in the quality of life of women after menopause(15). Therefore, the objective is to determine the validation of the instrument for the evaluation of climacteric and menopause in the first level of care.
METHODS
The research was developed using a quantitative approach, corresponding to the instrument validation design, prospective and cross-sectional. The participants were women with ages between 40 and 60 years, attended in four health establishments of the first level of care, located in the marginal urban area of Lima from November 2019 to February 2020.
176 patients participated who were selected at two moments using non-probability sampling. Inclusion criteria were patients who were treated for presenting symptoms related to climacteric-menopause in the medical office. At first, 40 patients were involved in the development of the pilot test, to verify the operation of the instrument and find the internal consistency analysis preliminarily. The second moment included 136 patients to determine the factor analysis and final internal consistency analysis of the instrument.
The procedure was carried out in three phases, according to the following description:
-
Phase 1: Initial
- Review Review of the literature: The scientific literature was reviewed in databases with an antiquity of ≤ 5 years, for which research carried out by Goldstein was taken into account S, Abernethy K and Potter B. et al.12,16,17; to identify the most important indicators regarding the management of climacteric-menopause
- Translation: The preparation of the instrument was carried out with the participation of four translators from English to Spanish and the subsequent reverse translation, generating the Spanish version of the instrument, taking as a reference the questionnaire "Menopause Quick 6 (MQ6)" developed by Dr. Susan Goldstein (12).
- The first version of the instrument: It contains six questions with dichotomous answers. The score that corresponds to a score ≥ of 4 affirmative responses recommends referring the patient for specialized management.
-
Phase 2: Testing
- The content validity was performed using the expert judgment technique, later the pilot test was carried out to estimate the reliability through the Kuder-Richardson formula and improve the design of the instrument for its application.
-
Phase 3: Psychometric properties
- The internal consistency analysis of the corrected instrument was applied to 136 patients. Then the exploratory factor analysis was carried out by applying the Kaiser-Meyer-Olkin index.
- The final version of the instrument: Made up of two sections. The first section includes sociodemographic data (age, marital status, education, work activity, harmful habits, lifestyle, cause of menopause, and body mass index. The second includes questions such as, Do you have any changes in your menstrual periods? Have you felt hot flashes or flushes? Do you have vaginal dryness, pain, or concern about sexual issues? Do you have a bladder problem or urinary incontinence? Has your quality of sleep decreased in recent months? And Has your mood worsened in recent months? Each question contains affirmative or negative answers. Obtaining 4 or more affirmative answers means that the patient must be referred for specialized management.
136 patients participated who met the inclusion criteria. The mean age corresponded to 48.6 ± 5.3 years, a mean for the number of children of 2.4 ± 1.4 and 27.8 ± 4.4 for the body mass index. About sociodemographic characteristics, the marital status of married corresponded to 40.4%. Likewise, 45.6% have secondary studies and 47.1% higher education, 69.1% are engaged in a work activity, 94.9% do not consume tobacco, 93.4% do not consume liquor regularly, 81.6 % stated that they did not practice sports, 66.9% maintained sexual activity and 96.3% presented physiological menopause.
RESULTS
Participaron 136 pacientes que cumplieron los criterios de inclusión. La edad media correspondió a 48,6 años ± 5,3, la media para el número de hijos a 2,4 ± 1,4 y 27,8 ± 4,4 para el índice de masa corporal. Con respecto a las características sociodemográficas, el estado civil de casada correspondió a 40,4%. Asimismo, 45,6% tienen estudios secundarios y 47,1% estudios superiores, 69,1% se dedica a una actividad laboral, 94,9% no consume tabaco, 93,4% no consume licor en forma habitual, 81,6% manifestó no practicar deporte, 66,9% mantiene actividad sexual y 96,3% presentó menopausia fisiológica.
Phase 1. Initial review
Criteria for the management of climacteric-menopause were identified and standardized through the use of menopausal hormone therapy without contraindications. The criteria were assessed by the presence of symptoms such as the presence of irregular menstruation, hot flashes and hot flashes, changes in the vulva-vagina considered as a genitourinary syndrome, sleep interruption as a consequence of the presence of hot flashes, and the presence of risk of presenting depression and irritability.
The initial translation of the instrument and the inverse translation did not present significant changes in formal aspects or meaning, which allowed the design of the first version of the instrument, which contains six questions with dichotomous answers and the visualization of the total score.
Phase 2: Trial
The content validity was performed using the expert judgment technique, for which 6 expert physicians were invited. The concordance index was 0.77 by Kappa index analysis. The recommendations issued by the experts were complementary in nature and with suggestions to improve the design for easy application.
With the incorporation of the recommendations, the pilot test was developed with the participation of 40 patients who met the inclusion criteria. Reliability was 0.79 using the Kuder-Richardson test. The average time for its application was approximately 2 minutes.
Phase 3: Psychometric properties
Internal consistency values were obtained by the Kuder-Richardson test of 0.69. It should be noted that 121 affirmative responses were obtained. Question 6 presented a greater relationship concerning the total score (Rho = 0.69). The analysis of the contribution made it possible to establish that no question should be eliminated (Table 1).
Table 1. Internal consistency analysis of the final version of the instrument (n = 136)
| Questions | Average | Correlation of total item | KR-20 with item deleted |
| Do you have any changes in your menstrual periods? | 0,59 | 0,66 | 0,61 |
| Have you felt hot flashes or flushes? | 0,61 | 0,64 | 0,61 |
| Do you have vaginal dryness or pain or concern about sexual issues? | 0,88 | 0,52 | 0,64 |
| Do you have a bladder problem or urinary incontinence? | 0,77 | 0,48 | 0,66 |
| Has your quality of sleep decreased in recent months? | 0,58 | 0,63 | 0,62 |
| Has your mood worsened in recent months? | 0,61 | 0,69 | 0,59 |
| Total Kuder-Richardson coefficient (KR-20) | 0,66 |
For factor analysis, the Kaiser-Meyer-Olkin test determined a result of 0.70. The questions explained 55.8% of the total variance, while the participants registered values between 0.40 (question 4) and 0.66 (question 6). For the factorial structure matrix and the Promax rotation, two factors were obtained. Questions 1, 2, 3 made up Factor 1 and questions 4, 5, 6 Factor 2. Likewise, the correspondence analysis to determine proximity allowed ratifying that the questions present a contribution to the structure of the instrument (Table 2).
Table 2. Exploratory factor analysis of the instrument (n = 136)
| Questions | Commonality | Factor 1 | Factor 2 |
| Do you have any changes in your menstrual periods? | 0,57 | 0,73 | 0,19 |
| Have you had hot flashes or flushes? | 0,60 | 0,76 | 0,13 |
| Do you have vaginal dryness or pain or concern about sexual issues? | 0,54 | 0,73 | 0,05 |
| Do you have a bladder problem or urinary incontinence? | 0,40 | 0,02 | 0,63 |
| Has your quality of sleep decreased in recent months? | 0,55 | 0,16 | 0,72 |
| Has your mood worsened in recent months? | 0,66 | 0,21 | 0,78 |
| Kaiser-Meyer-Olkin index (KMO) | 0,70 | ||
| Own value | 2,27 | 1,08 | |
| Variance (%) | 28,97 | 26,85 |
The version of the instrument contains six questions with dichotomous answers; affirmative (1) and negative (0). The total score is obtained by adding affirmative answers. Obtaining a score of 4 or more affirmative responses establishes that the patient must be referred for specialized management. Finally, the signature of the responsible physician and the date of care must be recorded (Figure 1).
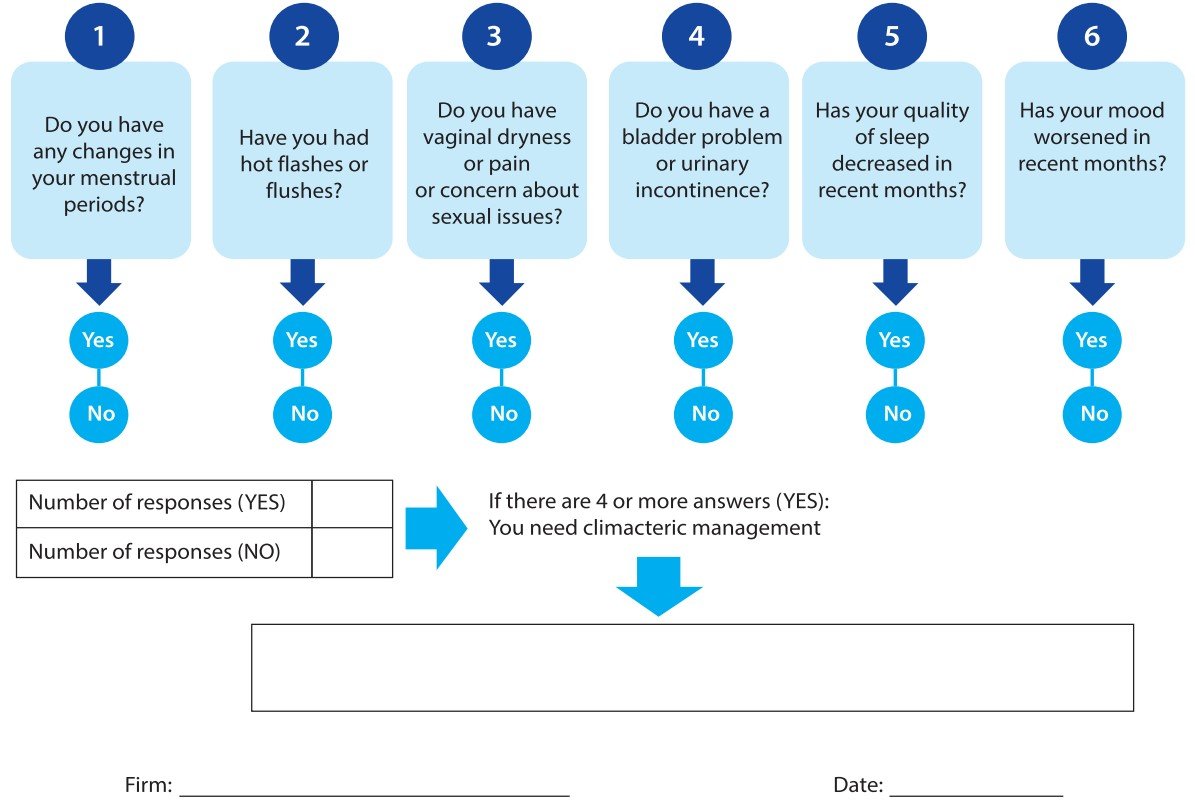
Figure 1. Taken from the questionnaire "Menopause Quick 6 (MQ6)" Menopause Quick 6 (MQ6) developed by Dr. Susan Goldstein. An efficient tool for the primary care management of menopause. Can Fam Physician. 2017 Apr;63(4):295-298. PMID: 28404707; PMCID: PMC5389763 (12).
DISCUSSION
The validation studies verify the validity and reliability of the design, as well as the construction of the instruments, taking into account the characteristics of the population. When instruments are translated into another language or they are designed taking into account various clinical recommendations from other countries, semantic changes of some specific terms related to the evaluation of the items may occur, due to cultural variability and grammatical didactics(18,19).
In the present study, the values obtained by the Kuder-Richardson test indicated good reliability. The value for each item (0.48 to 0.69) showed good internal consistency and the results were coherent and consistent with recommendations made by various investigations(12,20,21). Likewise, the item-total correlations of all items exceeded the minimum required level of 0.40, confirming the good internal consistency according to the reviewed research. The content validity and the pilot test allowed to demonstrate that the presence of four or more symptoms justifies the specialized management of the patients, being the mood with the greatest impact and that they require management according to the specific recommendations for the treatment of symptoms short term(4,16,22).
The instruments used in various investigations are of particular importance about the perception of symptoms related to female health and may differ in their conception and the design of an original instrument(23). The results of the exploratory factor analysis have succeeded in showing that the structure of the instrument is consistent for women during the climacteric. Likewise, the instrument made it possible to identify the most frequent symptoms, such as those related to vulvovaginal symptoms.
The design of the instrument contains six questions considered relevant for the start of specialized management, among which menstrual irregularities are included, since they are taken into account as imminent indicators of menopause. Various studies have reported the presence of vasomotor symptoms before the cessation of menstruation and these can have an average duration of 11.8 years; if the vasomotor symptoms appear after the last menstruation, these would have an average duration of 3.4 years. It is important to note that, currently, the use of hormone therapy is recommended for all women with the cessation of menstruation before the age of 45 and premature ovarian failure(3,12,24)..
Vasomotor symptoms affect most women during the menopausal transition, although their severity, frequency, and duration vary widely among women. These are reported by up to 85% of women and their average duration is approximately 5.2 years. The present study found that 64.7% of patients manifested the presence of vasomotor symptoms, which can be effectively treated with menopausal hormone therapy(4,11,25,26).
Likewise, vulvovaginal symptoms are very common, affecting up to 45% of women until after menopause(7). Currently, there is new terminology for genitourinary and sexual symptoms of menopause by the name genitourinary symptoms of menopause, due to the unfavorable effect on the vaginal mucosa, urethra, and sexual function(27,28). It should be noted that vaginal dryness or concern for sexual issues was reported by 68% of the patients, confirming the importance of evaluating this item, because it worsens as women age.
The decrease in sleep quality was reported by 60.2% of the patients. These results confirm that sleep problems increase when women are in the middle of the menopausal transition and can affect more than 50% of women. It is important to note that bad habits and mood disorders, particularly anxiety and depression, contribute to the presence of sleep difficulties. The clinical consequences of poor sleep quality include fatigue and daytime sleepiness, deterioration in the quality of life, which warrants specialized management through the use of menopausal hormone therapy(29,30), which confirms the relevance of including this symptom as an indicator in the instrument developed.
For many years, urinary incontinence has been associated with menopause, although the initial literature is not conclusive. Some research conducted in the 1980s reported that approximately 20% of 45-year-old women suffered from some degree of urinary incontinence, while other research reported up to 9% in premenopausal women and 12% in postmenopausal women. Although it is true, the questions asked only sought to obtain an answer about the presence or not of urinary incontinence or bladder problems, these corresponded to 50.7% of patients, contrary to those asked by Hording(31), where they could not identify a higher probability of preventing urinary incontinence among premenopausal women, which was confirmed by research carried out by Hagstad and Milson(32,33).
According to the analysis of the results, it was possible to show that care during the climacteric is crucial and should be considered the propitious moment to offer preventive medicine to preserve health and allow women the possibility of planning a life healthy, taking into account the increase in life expectancy.
The limited investigations do not report consistent data regarding the care of women during the climacteric in the first level of care, this seems to be due to various ethnic and social factors. However, it is estimated that more than a third of women require care from a general practitioner to treat and alleviate symptoms that women present after 50 years and before(20,34). For this reason, the main contribution of this study recommends the use of an easy-to-apply measuring instrument that in practice helps to identify women who have difficulties adapting to climacteric and require specialized intervention promptly.
Regarding the limitations, it can be mentioned that the sample used is not very large due to the refusal of some patients to participate in the research. Likewise, the nature of the questions in the instrument generates certain rejection and reservations of the patients to comment on them during the consultation. Likewise, medium-term symptoms and the presence of prevalent chronic diseases were not considered within the content.
CONCLUSION
In conclusion, the instrument is a short questionnaire, designed to assess and determine the assessment of climacteric and menopause at the first level of care. It has been validated in different stages, being the first study of this type carried out in Peru. It is an instrument with adequate content validity and internal consistency that will be useful for the management of climacteric and menopause in primary care facilities.
Declaration: We declare that the material contained in the manuscript has not been previously published or sent to another biomedical journal.
Author’s Contributions: The authors participated in the genesis of the idea, project design, data collection and interpretation, analysis of results, and preparation of the manuscript of this research work.
Funding: Self-financed.
Conflicts of interest: The authors declare they have no conflicts of interest in the publication of this article
Received: June 17, 2020
Approved: July 23, 2020
Correspondence: Juan P. Matzumura Kasano
Address: Av. Javier Prado Este 1066. Torre B Piso 7. San Isidro, Lima-Perú
Telephone: 999008897
E-mail: jmatzumura@yahoo.com
BIBLIOGRAPHIC REFERENCES
