CLINICAL CASE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2020 - Universidad Ricardo PalmaDOI 10.25176/RFMH.v20i4.3263
CARDIAC TAMPONADE AND INTRACEREBRAL HEMORRHAGE IN A CHILD WITH COVID-19: CASE REPORT
TAPONAMIENTO CARDIACO Y HEMORRAGIA INTRACEREBRAL EN UN NIÑO CON LA COVID-19: REPORTE DE UN CASO
Ricardo Enrique Rodríguez-Portilla1,2,a, Manuel Eduardo Munaico-Abanto1,3,a, Rosa Perlita Paredes-Zevallos1,a, Gaudi Amelia Quispe-Flores1,a
1Hospital Nacional Edgardo Rebagliati Martins-EsSalud, Lima-Perú.
2Profesor de práctica, residentado de pediatría, Universidad Científica del Sur, Lima-Perú.
3Profesor de fisiología, Universidad Nacional Mayor de San Marcos, Lima-Perú.
aPediatrician and pediatric intensivist.
The coronavirus disease 2019 (COVID-19) is a pandemic according to statements by the World Health Organization. It affects both the adult and pediatric population; however, most information published corresponds to the former, generating a knowledge gap for children. Pulmonary involvement seems to be the most frequent manifestation of the disease, although extrapulmonary conditions such as pericardial effusion and cerebrovascular diseases have been reported in adults. However, according to our review, none have been reported in children. We present the case of a 7-year-old male patient with a history of asthma and overweight who presented COVID-19, was hospitalized through the emergency service for pneumonia, and evolved with respiratory failure that required invasive ventilatory support. During his hospitalization, he presented cardiac tamponade and intracerebral hemorrhage with an unfavorable evolution and fatal outcome.
Key words: Cardiac tamponade; Cerebral hemorrhage; COVID-19; Child (Source: MeSH NLM).
RESUMEN
La enfermedad por coronavirus 2019 (COVID-19) es una pandemia según declaraciones de la Organización Mundial de la Salud. Afecta tanto a la población adulta como pediátrica; sin embargo, la mayor información publicada corresponde a los primeros, generando una brecha de conocimiento con respecto a los niños. El compromiso pulmonar parece ser la manifestación más frecuente de la enfermedad, aunque se han reportado afecciones extrapulmonares como derrame pericárdico y enfermedades cerebrovasculares en adultos; sin embargo, según nuestra revisión ninguna ha sido reportada en niños. Presentamos el caso de un paciente varón de 7 años con antecedentes de asma y sobrepeso que presentó la COVID-19, fue hospitalizado a través del servicio de emergencia por neumonía y evolucionó con insuficiencia respiratoria que requirió soporte ventilatorio invasivo. Durante su hospitalización presentó taponamiento cardiaco y hemorragia intracerebral con evolución desfavorable y desenlace fatal.
Palabras Clave: Taponamiento cardiaco; Hemorragia cerebral; COVID-19; Niño (fuente: DeCS BIREME).
The coronavirus disease 2019 (COVID-19), declared a pandemic by the World Health Organization in March 2020, affects both the adult and pediatric population. According to reports, 1.9% of all cases correspond to patients under 18 years of age with a fatality rate in those under 24 years of 0.07% and those under 14 years of 0.01%(1), (2). Although, a small proportion of them develop severe cases and as the pandemic has progressed, more hospitalizations have been reported in the Pediatric Intensive Care Units (PICU). The most likely to be hospitalized are children younger than 12 months or with comorbidities such as asthma (11.6%), cardiovascular disease (7.2%), and immunosuppression (2.9%)(3). Given the need to know more about this disease, we report the case of a child with COVID-19 who presented cardiac tamponade and cerebral hemorrhage during the evolution of his disease.
CASE REPORT
The patient is male aged 7 years and 10 months of age. He has a history of uncontrolled asthma and overweight with a body mass index at the 94th percentile, from a multi-family dwelling in crowded conditions in the Jesús María district of Lima. He was admitted through the Hospital National Edgardo Rebagliati Martins’ emergency service on April 20, 2020, referring to a two-day sick period. Sickness is characterized by fever, abdominal pain, non-explosive vomiting, non-productive cough, and erythematous macules on the lower limbs. There was no conjunctival injection, mouth lesions, cervical adenopathy, or edema in the distal area of the extremities. He was hospitalized and a serological test was taken to rule out COVID-19, obtaining positive Ig M and negative IgG.
During the first day of hospitalization, he evolved with progressive respiratory distress. He was admitted to invasive mechanical ventilation, after which he presented cardiorespiratory arrest, receiving advanced cardiopulmonary resuscitation for 6 minutes with a return of spontaneous circulation. Hemodynamic monitoring with ultrasound was performed, in which cardiac tamponade and left ventricular ejection fraction less than 50% was observed; It was drained, obtaining 180 cc of serous fluid from which samples were sent for cytochemical study and culture, which was later reported negative. An electrocardiogram was not performed due to the urgency of the case. The control chest X-ray showed diffuse and bilateral alveolar interstitial infiltrate in a more significant proportion than the initial one (Figure 1).
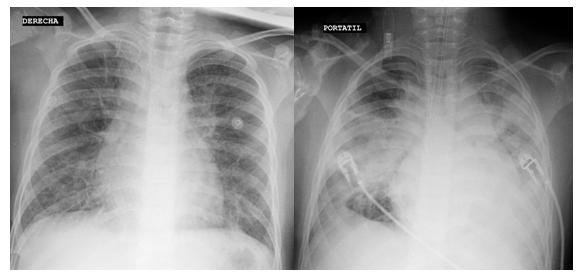
Figure 1. Chest X-rays. Left, on admission with interstitial infiltrates and bilateral bronchovascular reinforcement. Right, in control after intubation, with diffuse alveolar interstitial infiltrate.
The patient remained stable, with decreasing vasopressor and inotropic support until they were suspended. Laboratory tests such as the coagulation and lactate profile progressively fell into age-appropriate ranges. Also, ferritin, rheumatoid factor, antinuclear antibodies, antineutrophil cytoplasmic antibodies, and complement components 3 and 4 were within normal ranges (Table 1). Subsequent ultrasound controls showed a laminar pericardial effusion without hemodynamic compromise. A left ventricular ejection fraction greater than 55% and an indexed cardiac output greater than 3.2 liters per minute per square meter of the body surface, both values considered normal. The patient received treatment with hydroxychloroquine, in addition to antibiotic therapy with ceftriaxone and azithromycin, but did not receive anticoagulation. On the fourth day, the nasopharyngeal swab was taken polymerase chain reaction test with reverse transcriptase for SARS-CoV-2 (RT -PCR- SARS-CoV-2), which was negative.
Table 1. Clinical laboratory results.
| VARIABLE | Reference range (*) | Hospital day 0 | Hospital day 1 | Hospital day 2 | Hospital day 3 | Hospital day 4 | Hospital day 5 | |||||||||
| White blood cell count (x103/μL) | 5,0 – 14,5 | 20,76 | •• | 21,65 | 20,93 | 22,17 | 18,68 | |||||||||
| Neutrophil count (x 103/μL) | 1,8 – 8,0 | 18,64 | •• | 19,45 | 18,48 | 18,17 | 15,56 | |||||||||
| Lymphocyte count (x 103/μL) | 0,9 – 5,2 | 1,24 | •• | 0,65 | 1,172 | 1,7 | 1,78 | |||||||||
| Platelet count (x 103/μL) | 150 - 400 | 257 | •• | 250 | 96 | 274 | 295 | |||||||||
| Hemoglobin (g/dl) | 11,5 – 15,5 | 15,6 | •• | 12,9 | 11,1 | 11,7 | 10,5 | |||||||||
| pH | 7,35 – 7,45 | •• | 6,92 | 7,41 | 7,38 | 7,58 | 7,48 | |||||||||
| pCO2 (mmHg) | 35 - 45 | •• | 77 | 31,3 | 36,9 | 32,5 | 30,1 | |||||||||
| pO2 (mmHg) | 83 - 108 | •• | 70 | 69,7 | 119 | 73,6 | 89,5 | |||||||||
| Lactate (mmol/L) | 0,5 – 1,6 | •• | 7,9 | 1,4 | 1,1 | 1,5 | 0,9 | |||||||||
| Bicarbonate (mmol/L) | 22 - 26 | •• | 10,9 | 21,5 | 22,4 | 32,6 | 24,6 | |||||||||
| Urea (mg/dl) | 22 - 55 | 27,8 | •• | •• | 21,4 | 23,5 | 21,4 | |||||||||
| Creatinine (mg/dl) | 0,3 – 0,7 | 0,5 | •• | •• | 0,3 | 0,3 | 0,4 | |||||||||
| Albumin (g/dl) | 3,7 – 5,5 | 4,9 | •• | •• | 4,3 | 4,6 | 4,3 | |||||||||
| Aspartate aminotransferase (U / liter) | 15 - 40 | 28 | •• | •• | 75 | 65 | 30 | |||||||||
| Glutamate aminotranspheres (U / liter) | 10 - 35 | 13 | •• | •• | 39 | 49 | 40 | |||||||||
| C-reactive protein (mg / dl) | 0,0 – 1,0 | 12 | •• | •• | •• | 5 | 4,7 | |||||||||
| Procalcitonin (ng/ml) | <0,1 | •• | •• | •• | 3,8 | •• | •• | |||||||||
| Creatine kinase (U / liter) | 46 - 171 | •• | •• | •• | 2731 | 1912 | 471 | |||||||||
| Creatine kinase MB (ng/ml) | 0,0 – 6,0 | 48 | •• | 35,5 | •• | 8,9 | 2,7 | |||||||||
| Dimero D (ug/ml) | 0,0 – 0,5 | •• | •• | 9,7 | 4,4 | •• | 3,6 | |||||||||
| Prothrombin time (sec) | 10,5 – 13,0 | 14,3 | •• | 14,3 | 12,2 | •• | 12,1 | |||||||||
| Partial thromboplastin time (sec) | 25 - 37 | 34,46 | •• | 34,5 | 28,3 | •• | 25 | |||||||||
| Fibrinogen (mg/dl) | 200 - 400 | 468,5 | •• | 468 | 357 | •• | 346 | |||||||||
| Lactate dehydrogenase (U/liter) | 120 - 246 | 243 | •• | •• | 344 | •• | •• | |||||||||
| Ferritin (ng/ml) | 28 - 365 | •• | •• | 119 | •• | •• | •• | |||||||||
| C3 (mg/dl) | 90 - 160 | •• | •• | •• | 93 | •• | •• | |||||||||
| C4 (mg/dl) | 14 - 36 | •• | •• | •• | 9 | •• | •• | |||||||||
| Antinuclear antibody | Negative | |||||||||||||||
| Neutrophil cytoplasmic antibodies | Negative | |||||||||||||||
| Rheumatoid factor (IU/ml) | 0,0 – 14,0 | •• | •• | •• | 11,9 | •• | ||||||||||
| Pericardial fluid | 6000 cells - neutrophils 85% | Adenosine deaminase 15 U / L | ||||||||||||||
| Pericardial fluid culture | Negative | |||||||||||||||
| Blood culture | Negative | |||||||||||||||
| (*) Reference range in pediatrics | ||||||||||||||||
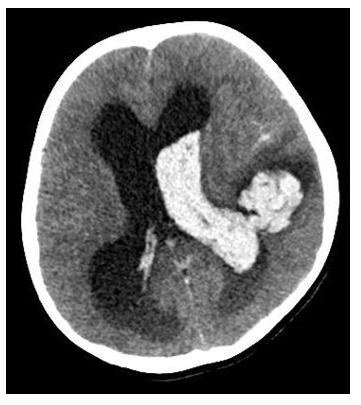
Figure 2. Multislice spiral tomography showing large left temporal intracerebral hemorrhage extending to the ventricle on the same side.
DISCUSSION
COVID-19 has a low incidence in children, although its clinical manifestations have been variable, every day, more cases of serious illness associated with SARS-CoV-2 infection are being reported(1)-(3).
The presence of cardiac tamponade in SARS-CoV-2 infection is infrequent in adults(4)-(7) and until the writing of this article, no cases have been reported in pediatric patients. In the case presented, a pericardial fluid with inflammatory characteristics was obtained. Still, the immunological tests, blood cultures, and cultures of the pericardial fluid were negative, eliminating the possibility that an autoimmune disease or bacterial infection is the cause of cardiac tamponade. This suggests that the etiology could be secondary to the SARS-CoV-2 infection.
The mechanism of action of SARS-CoV-2 is through the receptor for angiotensin-converting enzyme II (ACE II), which mediates its entry into cells and causes damage to various organs(8). Its entry into the cardiomyocytes is explained by the presence of these receptors on its surface. Also, the presence of the virus has been detected by SARS-COV-2 RT-PCR in pericardial fluid, which would make us suspect of possible cardiac involvement by the virus(9). In our case, the elevation of creatinine-phosphokinase fraction MB (CPK-MB), the pericardial effusion, in addition to the need for inotropic support, evidenced myocardial involvement. The study of the pericardial fluid for CRP-SARS-COV-2 was not possible as it was not standardized for this sample in our hospital.
On the other hand, in patients with COVID-19, manifestations in the Central Nervous System (CNS) have been described, such as encephalitis, demyelinating lesions, ischemic, and hemorrhagic vascular accidents(10)-(12). The neurological compromise would be explained by the presence of ACE II receptors in circumventricular organs and cerebrovascular endothelial cells that would allow their entry into brain tissue(13). Although it is not conclusive, the severity of the infection appears to be associated with the virus’s neurological invasion. In our case, intraparenchymal hemorrhage was described on the fifth day of hospitalization with no apparent cause to justify it, such as altered coagulation profile, platelet penia, and anticoagulants arteriovenous malformation or neoplasia; so it could be assumed as secondary damage from SARS-COV-2.
The report’s limitation was obtaining a negative RT-PCR result for SARS-CoV-2, despite having a serological test reactive to IgM. This can be explained by poor sampling technique in the nasopharyngeal swab in a patient with invasive mechanical ventilation. In the case of the Combined IgG-IgM Antibody Test Kit, Li et al. describe a sensitivity of 89% and specificity of 91% of the test and provide benefits such as greater ease of sample collection and more incredible speed in obtaining the results(14). Considering that the patient lived in a district with a high prevalence of confirmed COVID-19 cases, with compatible symptoms and a positive Ig M for the infection, the RT-PCR result was likely a false negative, as has already been reported in other cases(15).
In conclusion, cardiac tamponade and intracerebral hemorrhage, are rare events in patients with COVID-19, which have not been previously described in children. Although we cannot attribute the causality of these complications to SARS-CoV-2 infection, neither can their association be ruled out. This case report provides more information on the possible multisystem involvement of the virus, which allows for more knowledge about the clinical manifestations of this new infection.
Author’s Contributions: The authors participated in the genesis of the idea, project design, data collection and interpretation, analysis of results, and preparation of the manuscript of this research work. This manuscript was prepared using the CARE Guide by Ricardo Rodríguez, Manuel Munaico, Rosa Paredes, and Gaudi Quispe.
Funding: Self-financed.
Conflicts of interest: The authors declare that they have no conflict of interest.
Received: August 31, 2020
Approved: September 7, 2020
Correspondence: Ricardo Enrique Rodríguez Portilla.
Address: Jirón Huiracocha 1735 Departamento 603, Jesús María, Lima-Perú.
Telephone: +51 966915277
Email: ricardo.rodriguez@upch.pe
REFERENCIAS BIBLIOGRÁFICAS
