ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2021 - Universidad Ricardo Palma10.25176/RFMH.v21i4.4042
EFFECT OF ORCHIDECTOMIZED AND EXOGENOUS ADMINISTRATION OF TESTOSTERONE ON THE QUANTIFICATION OF PLATELETS IN MALE WISTAR RATS
EFECTO DE LA ORQUIDECTOMIZACIÓN Y LA ADMINISTRACIÓN EXÓGENA DE LA TESTOSTERONA EN LA CUANTIFICACIÓN DE LAS PLAQUETAS EN RATAS WISTAR MACHO
Milagros Fuentes -Vargas1, Lilian Lovón-Caso1, Marcia Paredes-Salazar1, Jheydi Cahuana-Gutierrez1, Ana Apaza-Choquehuanca1, Karla Elena Torres-Chávez2
1 Student of the Faculty of Human Medicine, UCSM, Arequipa Peru
2 Professor of the Faculty of Human Medicine, UCSM, Arequipa Peru
ABSTRACT
Introduction: Anabolic-androgenic steroids (AAS) modify the physiological functioning of the cardiovascular system and have possible effects on the origin of cardiovascular thrombosis. Objective: To determine the impact of the testosterone metabolite on platelet quantification in ORX rats with or without DHT replacement. Material and methods: 24 male 45-day-old Wistar rats underwent orchidectomy with a simple scrotal incision. At 2.5 months of age, the 24 rats were divided into 4 study groups. Half of the 12 male ORX rats received hormone replacement with dihydrotestosterone propionate (DHT) at a 2 mg/kg dose via subcutaneous injection for 7 days and the other half received a physiological solution (0.9% NaCl). The same occurred in the 12 non-ORX males (SHAM). After 7 days of the administration, blood was collected by orbital puncture, and platelet quantification was performed. Results: A significant difference (ANOVA, p < 0.005) was found between the 4 groups. When performing the Dunn Method, a significant difference (p < 0.05) was found in the endogenous administration of DHT between Sham rats and rats ORX + 0.9% NaCl but not with ORX + DHT. Conclusions: DHT induces an increase in platelet quantification inrats Sham and not in ORX rats with DHT, which may affect the increase in platelet quantification.
Keywords: Androgens; Testosterone; Anabolic; Hemostasis; Platelets, DHT. (source: MeSH NLM).
RESUMEN
Introducción: Los esteroides androgénicos anabólicos (EAA) modifican el funcionamiento fisiológico del sistema cardiovascular y tienen posibles efectos en el origen de trombosis cardiovascular. Objetivo: Determinar el efecto del metabolito de la testosterona en la cuantificación de plaquetas en ratas ORX con o sin reemplazo de DHT. Material y métodos: 24 ratas macho wistar de 45 días de edad fueron sometidas a orquidectomía con una incisión simple escrotal. A los 2 meses y medio de edad, las 24 ratas fueron divididas en 4 grupos de estudio. De las 12 ratas macho ORX, la mitad recibió reemplazo hormonal con propionato de dihidrotestosterona (DHT) a dosis de 2 mg / kg a través de una inyección subcutánea durante 7 días y la otra mitad recibió solución fisiológica (0.9% NaCl). De la misma manera ocurrió en los 12 machos no ORX (SHAM). Después de 7 días de administración, se extrajo sangre mediante punción orbital y se realizó la cuantificación de plaquetas. Resultados: Se encontró una diferencia significativa (ANOVA, p < 0.005) entre los 4 grupos. Al realizar el Método Dunn, se encontró una diferencia significativa (p < 0.05) en la administración endógena de DHT entre ratas Sham y ratas ORX + NaCl al 0.9% pero no con ORX + DHT. Conclusiones: DHT induce un aumento en la cuantificación de plaquetas en ratas Sham y no en ratas ORX con DHT, lo que puede afectar el aumento en la cuantificación de plaquetas.
Palabras Clave: Andrógenos; Testosterona; Anabólico; Hemostasia; Plaquetas; DHT (fuente: DeCS BIREME).
INTRODUCTION
Testosterone is the androgen derived from cholesterol with the highest presence in men, 95% of the total
circulating testosterone (6 to 7 mg/day). It is produced by Leydig cells in the testes (1,2).
The main effects of testosterone are two: androgenic and anabolic. The androgenic effects are
related to male sexual characteristics. The anabolic effects are responsible for the formation of
proteins, mainly the increase in muscle mass (3).
A group of chemically similar steroid hormones is anabolic-androgenic steroids (AAS) (4), which were created to minimize androgenic effects and maximize anabolic
ones by promoting skeletal muscle growth (5).
Among the therapeutic uses of AAS we have the restoration of muscle mass in the treatment of
cachexia associated with severe burns, kidney failure, and AIDS. They are also used as hormonal
substitutes in men with hypogonadism or low levels of circulating testosterone (3,6). However, self-administration to improve their physical conditions is
frequent in young male athletes and bodybuilders who want to improve their physical appearance by
increasing their muscle mass (7).
Currently, studies show that the use of AAS is frequent, and four out of five people are not
athletes and use them only for cosmetic purposes (8). Thus there is also
evidence that its use to improve performance and acquire more muscle is increasing Worldwide. Only in
the USA at least two million people use or have used AAS (9), on the other
hand, the prevalence in countries such as Norway, Argentina, and Poland were 3.6%, 6.5%, and 6.2%,
respectively (10-12), is older.
When the AAS are administered under medical prescription, they do not present risks, but in
athletes who abuse androgens, these serious side effects have been reported since 1988 (13). Among those most at risk is myocardial infarction, especially in
weightlifters (14). A stroke left ventricular hypertrophy and sudden cardiac
death are also found. Bile duct obstruction and an increased risk of tumors have been reported (3). All of these cases support the idea that exogenous androgens may be
thrombogenic (15).
Electrocardiographic studies show that very high doses of AAS modify the physiological
functioning of the cardiovascular system, which is why it is necessary to investigate the relationship
between endogenous testosterone concentration and its possible effects on the origin of cardiovascular
thrombosis (16). Testosterone therapy has also been associated with increased
expression of the thromboxane A2 receptor on platelets, in addition to increased platelet aggregation,
potentially creating an increased risk of thrombus formation (15).
The objective of this work was to determine the effect of the testosterone metabolite on
platelet quantification in ORX rats with or without DHT replacement.
METHODS
Animals
This study was carried out in male Wistar rats weighing 200 to 300 g obtained from the Animal Facility -
Universidad Católica de Santa María. The experimental procedures and protocols were approved by the
Ethics Committee of the Catholic University of Santa María and are in accordance with the IASP manual
for the study of pain in animals (17). The animals were housed in the
Bioterium, in metal cages (five rats/cage) in a room with controlled room temperature (23 ± 1 ° C) in a
12:12 light-dark light cycle, with food and water available ad libitum.
Orchidectomy (ORX)
45-day-old male Wistar rats underwent orchidectomy with a simple scrotal incision. The procedure was
performed under anesthesia induced by an intramuscular injection of ketamine (55 mg/kg) and xylazine
(5.5 mg/kg) mixture. Subcutaneous injection of ketoprofen (5 mg/kg) was used for postoperative analgesia
(18). Orchidectomy rats (ORX) were subjected to experimentation 4 weeks after
surgery. The sham rats operated underwent a surgical procedure similar to that of the ORX rats, except
that the gonads were not removed. (19,20)
Hormone administration
At 2 and a half months of age, the 24 rats were divided into 4 groups of study (6 rats in each group).
The group with 12 male ORX rats, consisted of 6 animals that received hormone replacement with
dihydrotestosterone propionate (DHT) at a dose of 2 mg/kg through a subcutaneous injection for 7 days
and, and 6 animals that received physiological solution (0.9% NaCl) (21).
Another parallel group with 12 non-ORX males was called rats SHAM (simulated), 6 of which received daily
subcutaneous administration of DHT propionate at a dose of 2 mg/kg and the other half physiological
serum (0.9% NaCl) for 7 days. The systemic administration of DHT, in ORX rats, was performed as a
replacement to match physiological levels compatible with that observed in shams (21). To determine if DHT induces an increase in platelet count, ORX or sham
DHT (2mg/kg) and ORX rats or sham NaCl (0.9%) were administered.
RESULTS
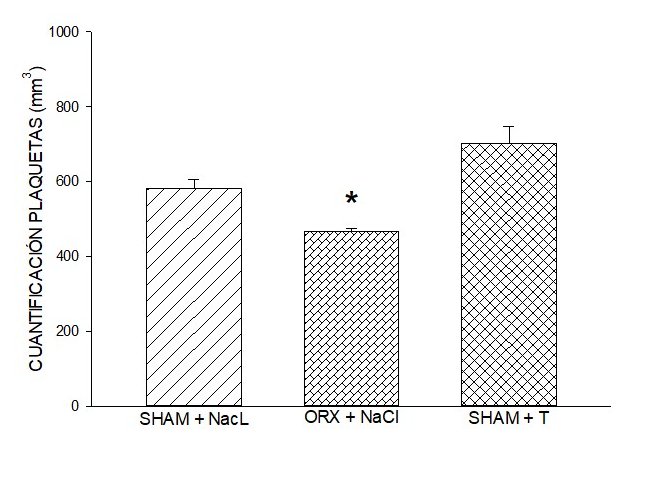
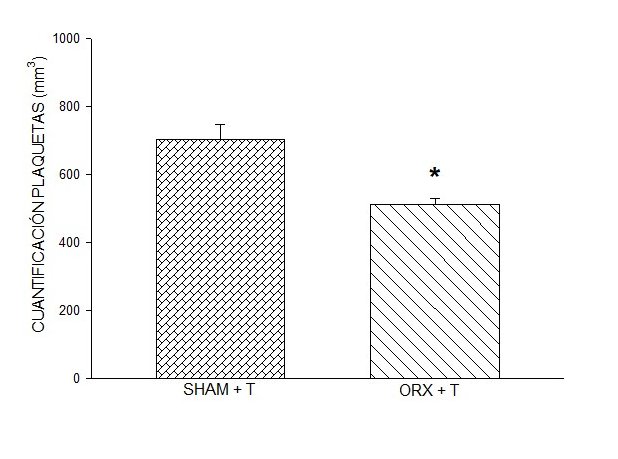
One-way ANOVA was used to determine if there were significant differences between the groups in Figure 1, the level of statistical significance was P < 0.005. The Dunn Method
post hoc test was used to determine the basis for significant differences. The T-test or the Rank Sum
Test were used when appropriate; the level of statistical significance was P < 0.04. Data are
presented in figures as means ± SEM. The Sigma Stat program was used for statistical analysis.
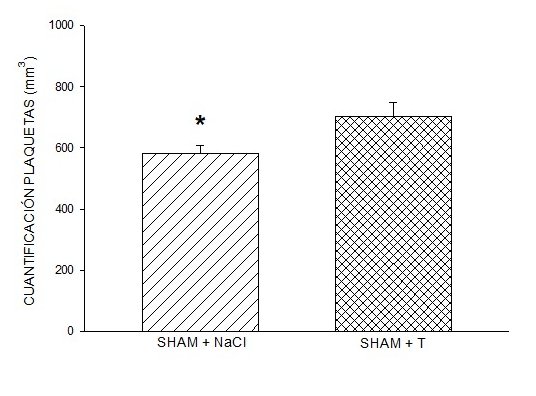
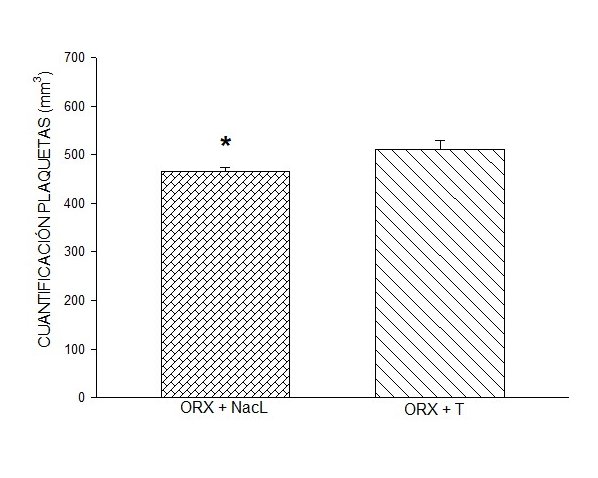
DHT administration in male ORX rats induced a quantification response similar to that induced by
0.9% NaCl (saline). However, the quantification response induced by the administration of DHT in sham
males was significantly greater than that induced by 0.9% NaCl (Figure 1), one-way
ANOVA, p < 0.005, and test to compare two groups (p < 0.04). The quantification response found
similar endogenous testosterone results in ORX males and males sham with 0.9% NaCl, which showed that
the DHT dose was physiological concentration (failed T-test, p < 0.05). The statistical difference
between endogenous DHT administration between rats sham and ORX male rats (Dunn method, p < 0.05)
suggests that the impact of the prolonged reduction in physiological testosterone concentration, through
ORX, on platelet expression TXA2 affects upregulation of platelets, suggesting controlled endogenous
testosterone concentration.
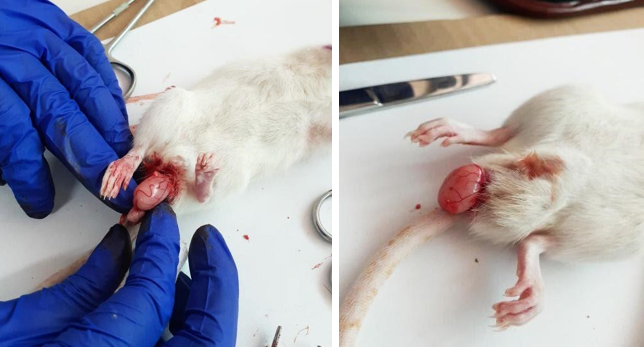
A simple scrotal incision was made under anesthesia, the testicle was identified, the spermatic cord was tied, and the cut was made to avoid bleeding. The same was done in the other gonad (19)
DISCUSSION
This study shows a significant difference between the DHT replacement group and the other comparison
groups. Blood platelet levels decrease in situations such as ORX induced by endogenous testosterone
decrease, in addition, the blood platelet level decreases in young ORX rats that received physiological
saline when compared to the group of rats that received exogenous DHT. A double-blind study by Ajayi
coincides with a significant increase in platelet aggregation, with anabolic androgenic steroids
contributing to a thrombotic state (22).
Karolczack et al., Showed that the effect of sex steroids in vitro, where testosterone is
stimulated by collagen or arachidonate, has the power to inhibit platelet aggregation in people in the
60-65 age group, including men and women (23), a study that evaluates the
modulation of TXA2, through the administration of testosterone, showed that the level of TXA2 protein
synthase increases in cerebral blood vessels (24); however, more studies are
required to rule out that there is another indirect effect causing this action. In comparison with the
study by Campelo et al., it was obtained as a result that testosterone inhibits platelet aggregation,
indicating that it is a nitric oxide-dependent effect. Endothelial (25). In
summary, this study suggests that the administration of steroid anabolic androgenic did not affect
platelet regulation because, after castration, the circulating testosterone concentration is associated
with a significant decrease in platelet receptor density TXA2 (26).
In another experiment, it was seen that the supraphysiological administration of testosterone
inhibits fibrinolytic activity and the bleeding time is significantly prolonged; however, the platelet
aggregation induced by ADP was unchanged (27). Studies currently suggest that
the consumption of anabolic androgenic steroids is associated with thromboembolism and increases its
thrombotic risk since they inhibit the production of a platelet aggregation inhibitor prostacyclin,
generating hypercoagulable states (28), also presenting cardiomyopathies,
hypertrophy cardiac among others (29).
Some studies conclude that there are more harmful events in men who had hormone replacement
therapy or consumption of anabolic androgenic steroids (30-33); however,
there are others where they found neutral and even beneficial effects (34,35).
Given the diversity of results, this due to different factors such as the androgen prototype
used, the number of doses, the time of treatment, the form of administration and the type of laboratory
tests that exist for its evaluation, is that the FDA warns of the consequences of using this type of
substance without medical consultation or indication, approving only its use in cases of hormone
replacement treatments (36). The misuse of anabolic androgenic steroids can
lead to dependence in a physical and psychological context. (37)
Various studies indicate a higher prevalence of anabolic-androgenic steroids in young people
aged 15-24 years by 54% (11). The population that consumes these androgens
mustn’t abuse the concentrations, given that long-term adverse effects may occur. Our research suggests
expanding and continuing with studies to elucidate the empirical relationship between the use of these
anabolic androgenic steroids and the consequences on the body of this abuse, as the number of people who
use these substances continues to increase.
CONCLUSION
This study shows that DHT increases platelets in Wistar rats when testosterone is administered at supraphysiological levels, suggesting a probable TXA2 pathway. Subsequent studies hope to elucidate this likely mechanism, using TXA2 inhibitors to better clarify through up-regulation of platelets.
Recognition: Special recognition is given to the personnel who work in the Animal Farm,
for their great support in this project, and to UCSM for promoting research in its students.
Authorship contributions: The authors participated in the conception of the study,
collection, analysis and discussion of the data. participated in the preparation of the
manuscript, bibliographic search and approval of the final version of the manuscript.
Financing: This project was carried out with a fund won in a Research Projects contest at
UCSM, Arequipa-Peru.
Conflicts of Interest: None declared by the authors.
Received: July 19, 2021
Approved: September 02, 2021
Correspondence: Karla Elena Torres Chavez
Address: Campus central Urb. San José s/n Umacollo Arequipa - Perú
Telephone: +51 950 342 755
E-mail: emailktorres@ucsm.edu.pe
BIBLIOGRAPHIC REFERENCES