ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2021 - Universidad Ricardo Palma10.25176/RFMH.v21i4.4265
ECHOCARDIOGRAPHIC PARAMETERS RELATED TO PULMONARY ARTERIAL HYPERTENSION IN PATIENTS WITH CHRONIC MYELOID LEUKEMIA UNDER DASATINIB TREATMENT
PARÁMETROS ECOCARDIOGRÁFICOS RELACIONADOS A HIPERTENSIÓN ARTERIAL PULMONAR EN PACIENTES CON LEUCEMIA MIELOIDE CRÓNICA EN TRATAMIENTO CON DASATINIB
Poot-Noh Karla J.1,a , Hernández-Jiménez Ernesto1,a , Juárez-Santiesteban María del R. 2,b , Zagoya-Martínez Patricia 2,c , Montiel-Jarquín Álvaro J.2,d , García-Galicia Arturo 2,e,g , Herrera-Solano David E. 3,f
1. Departamento de Cardiología, Hospital de Especialidades, Centro Médico Nacional General de Div. Manuel Ávila Camacho, Instituto Mexicano del Seguro Social, Ciudad de Puebla, Puebla-México.
2. Dirección de Educación e Investigación en Salud, Hospital de Especialidades, Centro Médico Nacional General de Div. Manuel Ávila Camacho, Instituto Mexicano del Seguro Social, Ciudad de Puebla, Puebla-México. 3. Universidad Popular
Autónoma del Estado de Puebla-México.
a. Cardiology Specialist Physician.
b. Allergology Specialist Physician.
c. Hematology Specialist Physician.
d. Specialist in General Surgery, Master in Medical Sciences and Research.
and. Specialist in Pediatrics. Master in Medical Sciences and Research.
f. Medical Intern of the Social Service.
ABSTRACT
Introduction: The use of dasatinib in patients with CML has improved life expectancy and follow-up with transthoracic echocardiography (ECOTT) for early detection of PAH allows modifications to the treatment. Objective: To determine the echocardiographic parameters and echocardiographic probability for PAH in patients with CML treated with dasatinib. Methods: Correlation, cross-sectional, retrospective, single-center study; patients with CML treated with dasatinib were included. Spearman and Pearson correlation was used. Results: 16 patients were analyzed, mean age 53.5 years; 62.5% men, 37.5% women. The dasatinib dose was 50 mg / day in 18.7%, and 100 mg / day in 81.2%, mean pulmonary arterial pressure (mPAP) 26.3 mmHg, mean maximum tricuspid regurgitation velocity (VmxRT) 2.9 m / s, mean pulmonary artery systolic pressure (PSAP) 41 mmHg. 56.2% had right ventricular diastolic dysfunction (RVDD). 43% were categorized as low probability for PAH, 18.7% intermediate, and 37.5% as high. Relationship between PAPm and VmxRT with p = 0.012. Relationship between mPAP and RV diastolic function, with p = 0.002. Relationship between probability for PAH and mPAP, with p = 0.008. Conclusion: The echocardiographic parameters PAPm, VmxRT, PSAP, DDVD and echocardiographic probability for PAH are useful and necessary for the diagnosis of PAH. The determination of all these parameters should be carried out early and as a follow-up, since a considerable positive relationship was found for each one with the presence of PAH, which is not dependent on the treatment time or the dose of dasatinib.
Keywords: Pulmonary arterial hypertension, Leukemia, Dasatinib, Echocardiogram (Source: MeSH NLM).
RESUMEN
Introducción: La leucemia mieloide crónica (LMC) es un desorden clonal de células madre hematopoyéticas, predomina en el género masculino entre 55 y 65 años. El ecocardiograma transtorácico (ECOTT) para detección temprana de hipertensión arterial pulmonar (HAP) permite hacer modificaciones al tratamiento con dasatinib. Objetivo: Determinar los parámetros ecocardiográficos y la probabilidad ecocardiográfica para HAP en pacientes con LMC tratados con dasatinib. Métodos: Estudio de correlación, transversal, retrospectivo, unicéntrico; se incluyeron pacientes con LMC tratados con dasatinib. Se utilizó correlación de Spearman y Pearson. Resultados: Se analizaron 16 pacientes, edad promedio 53,5 años; 62,5% hombres, 37,5% mujeres. La dosis de dasatinib fue 50 mg/día en 18,7%, y 100 mg/día en 81,2%, presión media de la arteria pulmonar (mPAP) 26,3 mmHg, velocidad máxima de regurgitación tricuspídea (VmxRT) media 2,9 m/s, presión sistólica de arteria pulmonar (PSAP) media 41 mmHg. El 56,2% presentó disfunción diastólica del ventrículo derecho (DDVD). Se categorizaron 43% en baja probabilidad para HAP, 18,7% intermedia y 37,5% en alta. Relación entre mPAP y VmxRT con p=0,012. Relación entre mPAP y función diastólica del ventrículo derecho (FDVD), con p=0,002. Relación entre probabilidad para HAP y mPAP, con p=0,008. Conclusión: Los parámetros ecocardiográficos mPAP, VmxRT, PSAP, DDVD son útilesy la probabilidad ecocardiográfica para HAP deben realizarse de forma temprana y como seguimiento, ya que se encontró relación positiva con la presencia de HAP, la cual no es dependiente del tiempo de tratamiento ni de la dosis de dasatinib.
Palabras Clave: Hipertensión arterial pulmonar, Leucemia; Dasatinib, Ecocardiograma (Fuente: DeCS BIREME).
INTRODUCTION
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell disorder; is characterized by the presence of Philadelphia chromosome (Ph) with reciprocal translation between chromosomes 9 and 22, resulting in BCR-ABL, which encodes a constitutively
active tyrosine kinase (TK)(1,2). It predominates in male sex (ratio 1.6: 1). The mean age at diagnosis is between 55-65 years old. Diagnosis depends on finding the abnormal Philadelphia chromosome t (9;22) (q34;q11.2) (3).The
clinical use of tyrosine kinase inhibitors(TKI) was selectively directed at blocking the enzymatic activity of BCR-ABL, imatinib being the first available drug. (4). The need to improve existing therapies gave
rise to a second generation of ITK, with drugs such as dasatinib. Dasatinib is 325 times more potent than imatinib. The FDA approved the use of this drug for the treatment of CML in Philadelphia chromosome positive patients (5,6).
Dasatinib inhibits multiple TKs, including BCR-ABL1, platelet-derived growth factor receptor β, and Src family kinases (7,8). The adverse reactions of dasatinib were discovered during clinical studies leading to its approval. From the phase 1 clinical trial, grade 3 and 4 toxicities were observed. In the phase III
DASISION clinical trial (2016), pleural effusion (associated with elevated pulmonary arterial pressures) was reported in 28% of cases and 14 ( 5%) patients presented PAH (9-11).
Pulmonary Hypertension (PH) results from flow restriction in pulmonary artery circulation, culminating in right heart failure. It is characterized by a mPAP ≥25 mmHg at rest (normal at rest 14 ± 3). Patients presenting in the mPAP
range between 21-24 mmHg should be followed when they are at risk of developing PAH (12,13). TKI-induced PAH has a low incidence and the dose of dasatinib does not appear to be correlated with
the risk of developing PAH(14).
Regarding the mechanism of PAH, induced by dasatinib, it is known that it inhibits the Src family of kinases that participate in cell proliferation and regulate AP smooth muscle tone through the potassium channel-1 pathway sensitive
to TWIK-2. It leads to depolarization of smooth muscle cells, vasoconstriction, and increased pulmonary arterial pressure; at the same time, it has an indirect association with the production of nitric oxide and prostaglandins responsible
for vascular relaxation, so that by inhibiting the Scr signaling pathways an imbalance between dilation and vasoconstriction occurs (15,16).
In the diagnosis of PAH by echocardiography, right heart catheterization is the technique of choice, being an invasive procedure with multiple complications. Because the symptoms of PAH are nonspecific and the physical signs are
subtle, the diagnosis is usually late and in advanced stages of the disease, for this reason it is necessary to have non-invasive and low-risk techniques that have a high rate of efficacy to detect PAH before irreversible physiological
damage occurs. By transthoracic echocardiography (ECOTT), direct and indirect signs of elevated pulmonary arterial pressure (PAP) can be obtained, which is useful for the diagnosis and prognosis of PAH (19, 20). The tricuspid regurgitation velocity (TRV) parameters used to estimate pulmonary artery systolic pressure (PASP) since there is a good correlation with PAP measured by right heart catheterization.
Echocardiographic diagnosis of PAH is probably when VRT> 3.4 m / s. It is possible when the VTR is between 2.9-3.4 m / s or when TRV ≤ 2.8 m / s with additional variables that suggest PAH (dilation of the right chambers, hypertrophy
of the right ventricle, dilation of the pulmonary artery trunk)
(21,22). By color Doppler echocardiography, mean pulmonary artery pressure (mPA) can be estimated using acceleration time in the ventricular outflow tract (23).
The use of tissue Doppler to measure (FDVD) is considered useful and characteristic in PAH. In 2005, they describe a significant increase in the isovolumic relaxation time (IVRT); currently there is validation of the correlation of IVT
with PAH, PSAP and diastolic dysfunction (24). In 2017, Skride et al. (25), presented a case report, a 67-year-old man with CML treated with dasatinib. After treatment, she presented
pleural effusion; Severe PAH with 53 mmHg mPAP was confirmed by ECOTT and by right heart catheterization. Given these findings, dasatinib was discontinued. A one-month follow-up study showed a significant improvement in mPAP of 34 mmHg.
In 2016, Morishita et al. (26), presented a report of a patient with CML who had been administered dasatinib for three years; 34 months later, the patient presented exertional dyspnea, there was no increase in
pleural effusion (which she had previously presented); PAH was suspected by ECOTT. PAH was then confirmed by cardiac catheterization.
In 2016, Minami et al. (27) presented a comparative analysis of PAH in patients treated with different TK inhibitors; ECOTT was used to assess the incidence of PAH in 105 CML patients treated with imatinib
(n = 37), nilotinib (n = 30) or dasatinib (n = 38), using the mean peak tricuspid regurgitation gradient (TRPG). A TRPG> 31 mmHg suggested possible PAH in nine of 105 patients: one (2.7%) treated with imatinib, three (10.0%) with nilotinib,
and five (13.2%) with dasatinib. These results suggested that treatment with dasatinib, imatinib and nilotinib may be associated with subclinical PAH, concluding that non-invasive echocardiography is useful for the detection of PAH in
patients treated with second generation TKI.
In 2016 Jin et al. (28) presented the case of a 55-year-old man with CML treated with dasatinib at a dose of 100 mg per day. After 36 months of treatment, he presented with dyspnea, fatigue and edema.
ECOTT was performed in which growth of the right ventricle and atrium was documented, with PSAP estimated at 115 mmHg and pericardial effusion. He presented improvement when stopping dasatinib treatment, documenting a reduction in PSAP
37-82 mmHg measured on three occasions. In 2016 Nagasaki et al. (29) reported the case of a 59-year-old man, with CML treated for five years, at a dose of 100 mg per day. He presented dyspnea with deterioration
of functional class. ECOTT was performed finding PAH data, with PSAP of 80 mmHg and a PAPm of 29 mmHg. Treatment was discontinued, showing a decrease in PSAP of 51 and 40 mmHg at the first month and year respectively. In 2015 Hong et al.
(30), reported two cases of CML patients treated with dasatinib. One case was a 43-year-old man, who after 69 months of treatment presented with dyspnea, performing ECOTT with PSAP of 92 mmHg, enlargement of
the right ventricle and atrium, as well as mild pleural effusion. The second case was a 52-year-old man, who presented dyspnea, ECOTT was performed with PSAP 71 mmHg, mild pleural effusion. Both cases presented improvement and decrease
in PSAP after discontinuation of dasatinib.
The use of dasatinib in patients with CML has improved life expectancy; however, drug toxicity (PAH) has been documented and may become irreversible. In the early stages of PAH, most patients can be asymptomatic and can be detected
by ECOTT. Performing an echocardiogram is inexpensive and takes a relatively short time. It focuses on measuring PSAP and PAH-related findings. Furthermore, it is important to measure FDVD, since the right heart chambers are affected in
the long term. The follow-up of patients treated with dasatinib, by means of ECOTT is important for the early detection of PAH and to be able to make modifications to the treatment.
Therefore, the objective of this study was to determine the useful echocardiographic parameters to diagnose PAH in patients with CML under treatment with dasatinib, in order to make an early diagnosis and follow-up.
METHODS
An observational, cross-sectional, retrospective, retrolective, single-center and homodemic correlation study was carried out. The design and type of sampling was deterministic. Patients from the Hematology Service with a diagnosis of CML under treatment
with dasatinib were studied. Patients older than 18 years and younger than 80 years were included. Patients with incomplete echocardiographic data were excluded. A total population of 31 patients was obtained, of which 15 were excluded
because they did not have an echocardiographic study in the work base.
The study data were obtained from the clinical record and from the syngo SC2000 workplace database located in the cardiology office. The right ventricular diastolic function was measured by ECOTT, mPAP, VmxRT, PSAP, and the echocardiographic
probability for pulmonary hypertension was determined; The dasatinib dose indicated in each patient was identified, as well as the treatment time. An Excel database was prepared and it was established whether or not there is PAH and what
echocardiographic findings are related. PAH was considered from mPAP greater than 25 mmHg. Echocardiographic parameters related to PAH were considered as VmxRT greater than or equal to 2.8 m / s, PSAP greater than 35 mmHg and DDVD. The
data were analyzed with descriptive statistics, using measures of central tendency and dispersion, using parametric and non-parametric statistical tests (Spearman and Pearson correlation). The analysis was performed using Excel version
16.39 and SPSS version 26. Human, material and financial resources were provided by the main investigators of the study.
The study was approved by the Comité Local de Investigación en Salud. This study was designed in accordance with the Reglamento de la ley General de Salud (reglamento de la Ley General de Salud en materia de investigación para
la salud). It adhered to the ethical standards drawn up in Helsinki in 1972 and modified in 1989. It was submitted for evaluation by the local research committee of our Medical Unit. The privacy of the patients studied was protected.
RESULTS
A total of 16 patients were analyzed (Table 1 and 2 for statistical analysis), the mean age was 53.5 years, with a SD of 11.7. Regarding gender, 10 patients were male (62.5%) and six female (37.5%). Of the total number
of patients, two (12.5%) had concomitant diagnoses of type 2 diabetes mellitus (DM2), chronic arterial hypertension (HAC) and chronic kidney disease (CKD); one patient (6.2%) had isolated stage 3a CKD, no ischemic heart disease or structural
heart disease was found; 13 patients (81.3%) did not present comorbidities. This is different from the study published by Minami et al.
(27) in 2017, who among the comorbidities reported a patient with chronic ischemic heart disease, without reporting other comorbidities. These results in the patients studied were due to the prevalence in our
setting of DM2 and hypertension and the presence of CKD as a complication associated with such comorbidities. Regarding the dose of dasatinib, three patients were administered 50 mg per day (18.7%), and in 13 patients 100 mg per day (81.2%).
As for mPAP, it had a mean of 26.3 mmHg, SD 13.63. With the variable VmxRT, a mean of 2.9 m / s, SD 0.51, was obtained. Regarding PSAP, a mean of 41 mmHg, SD 14.02, was obtained. Of the total number of patients, nine patients (56.2%) were
found with DDVD and seven patients (43.7%) with normal FDVD. In respect of probability for PAH, seven patients (43%) were categorized as low probability, three patients (18.7%) for intermediate probability, and six patients (37.5%) for
high probability. Of the patients with a history of DM2, HAC and CKD, two patients (100%) were found with PAH and DDVD, as well as Vmx RT> 2.8 m / s and PSAP> 35 mmHg. The patient with isolated CKD was found with DDVD, Vmx RT> 2.8 m /
s and PSAP> 35 mmHg (classified as high echocardiographic probability for PAH). No report similar to those obtained was found in previous studies.
The relationship between the treatment time with dasatinib and the mPAP figures had a value of r = -0.315 with a value of p = 0.235. The relationship between mPAP and dasatinib dose had a value of r = 0.122 with a value of p =
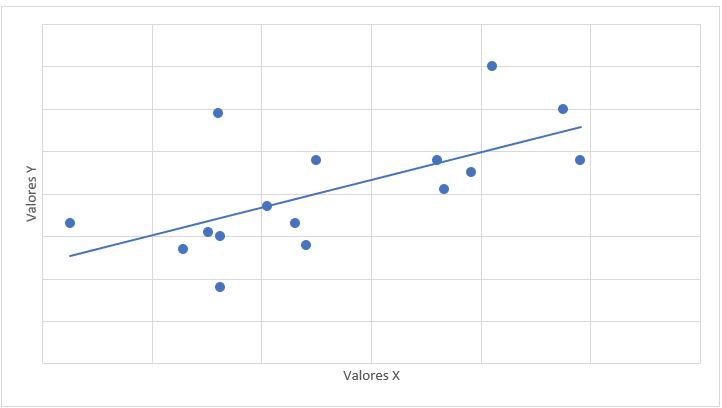
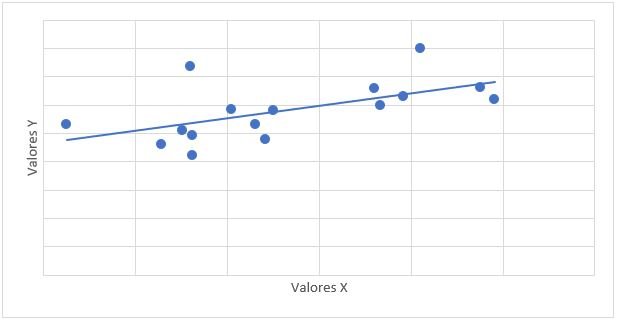
0.653. The relationship of mPAP with PSAP had a value of r = 0.647 and a value of p = 0.007 (Graph 1). The relationship between mPAP and VmxRT had a value of r = 0.612 and a value of p = 0.012 (Graph 2).
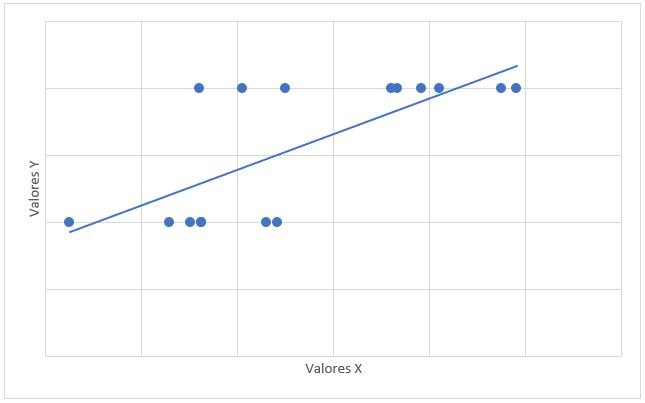
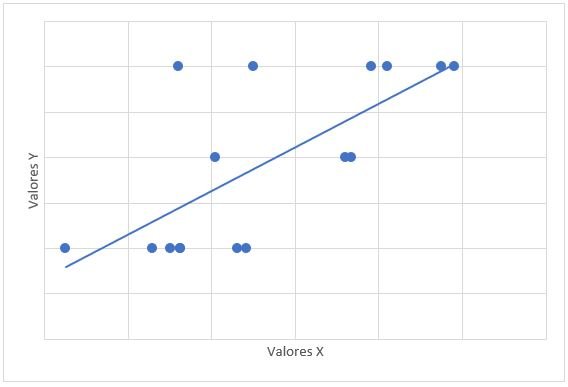
The relationship between mPAP and FDVD had a value of r = 0.708 and a value of p = 0.002 (Graph 3). The relationship between the probability for PAH with mPAP had a value of r = 0.706 and a value of p = 0.008 (Graph 4).
Table 1. Statistical analysis of quantitative variables.
| Variables | Mean | Minimum | Maximum | Standar deviation |
|---|---|---|---|---|
| Time | 146,2 | 14,7 | 381,4 | 101,26 |
| PASP | 41 | 18 | 70 | 14,02 |
| Vmx TR | 2,9 | 2,1 | 4.0 | 0,51 |
| mPAP | 26,3 | 2,5 | 49,1 | 13,63 |
Table 2. Statistical analysis of quantitative variables: dasatinid dose, FDVD and probability for PAH.
| Dosis de dasatinib | No. patients | Percentaje | Reason | Rate | Sampling error | 95% confidence interval |
|---|---|---|---|---|---|---|
| 50 mg | 3 | 18,8% | 0,23 | 23 | 9,75 | 0,41<18,75<37,86 |
| 100 mg | 13 | 81,3% | 4,33 | 433 | 9,75 | 62,14<81,25<100 |
| Total | 16 | |||||
| DFRV | No. patients | Percentaje | Reason | Rate | Sampling error | 95% confidence interval |
| Normal | 7 | 43,7% | 1 | 100 | 12,4 | 19,3<43,7<68 |
| Dysfunction | 9 | 56,3% | 1,29 | 129 | 12,4 | 32<56,3<80,6 |
| Total | 16 |
| Dosis de dasatinib | No. patients | Percentaje | Reason | Rate | Sampling error | 95% confidence interval |
|---|---|---|---|---|---|---|
| Probability for PAH | No. patients | Percentaje | Reason | Rate | Sampling error | 95% confidence interval |
| low | 7 | 43,7% | 2,3 1,16 | 230 116 | 12,4 | 19,4<43,7<68 |
| Intermediate | 3 | 18,8% | 0,42 0,5 | 42 50 | 9,76 | 0,32<18,8<37,9 |
| High | 6 | 37,5% | 0,85 2 | 85 200 | 12,1 | 13,8<37,5<61,2 |
| Total | 16 |
|
|
|
|
DISCUSSION
CML is a hematopoietic clonal disorder that can progress to an accelerated phase and death. The use of dasatinib has shown a good hematological and cytogenic response, however it is associated with PAH, which is the result of excessive abnormal proliferation
of smooth muscle. The development of PAH conditions involvement of the right heart chambers (diastolic dysfunction), which confers a worse prognosis. Echocardiographic measurements to evaluate the presence of PAH are varied, being the
mPAP obtained by acceleration time of RVOT one of the most reliable. Other echocardiographic values, when mPAP is not available or is not considered reliable, suggest a probability of PAH, classifying the probability as high, intermediate,
or low. In addition, through the use of pulsed and tissue Doppler, it is possible to obtain the FDVD, which is not dependent on the measurement obtained from mPAP.
Of the total of patients studied with CML treated with dasatinib, nine (56.2%) present echocardiographic changes related to PAH, of which seven patients have an estimated mPAP> 25 mmHg and two patients have an intermediate and
high probability for PAH (with mPAP < 25 mmHg).
As additional findings, five patients had mild pericardial effusion and one also presented pleural effusion, four presented mPAP greater than 25 mmHg and the one with mPAP less than 25 mmHg presented intermediate probability for
PAH. All five had DDVD, a PSAP> 35 mmHg and VmxRT> 2.8 m / s. Two patients in the sample without pericardial effusion presented isolated pleural effusion. No previous record was found of the study of pericardial or pleural effusion in
patients with CML treated with dasatinib.
Other echocardiographic findings were the presence of mild valvular disease: three patients with only tricuspid regurgitation (TR); four patients with only mitral regurgitation (MR), of which one did not present PAH data; two patients
with MI and mild TI, of which one did not present PAH data. Regarding these valvular heart diseases, the algorithm was used to determine the filling pressures of the left ventricle (LV) and diastolic dysfunction, obtaining from patients
with only TI and PAH two patients with grade I diastolic dysfunction with normal left atrial (LA) pressure and an indeterminate patient; of the patients with MI only, one patient with grade II diastolic dysfunction with increased LA pressure
and two patients with grade I diastolic dysfunction, with normal LA pressure; the patient with MI and TI together with PAH was classified as indeterminate.
There are no previous studies of valvular heart disease in CML patients treated with dasatinib. These results are considered comorbidities, since two patients were not found with PAH, only in one was an increase in filling pressures
that influence the presence of PAH due to the presence of grade II diastolic dysfunction and not due to the presence of mild MI. , four patients had normal filling pressures, and two were indeterminate. At the end of the study, the death
of three patients included in the sample was reported. One patient died due to progression of the disease to the blast phase, and two patients due to complications associated with pre-existing comorbidities (DM2 and CKD). The three patients
complied with mPAP ≥ 25 (PAH), presented DDVD, PSAP> 35 mmHg, VmxRT> 2.8 m / s, and high probability for PAH. No previous studies were found on mortality from PAH in CML patients treated with dasatinib. The results of the study are due
to the presence of comorbidities that influence the progression of PAH. The relationship between the dasatinib treatment time and the mPAP figures of the studied patients was found using the Spearman equation; a value of R = -0.315 (mean
negative correlation) was obtained, resulting in a non-significant p.
In the study carried out by Minami et al. (27), in 2017, a weak negative relationship was found with a non-significant p. In the study carried out, there is a relationship between the dose and the presence
of PAH, but no statistical significance was found, that is, the increase in the dose is not necessarily accompanied by an increase in mPAP. Regarding the relationship of mPAP with PSAP, a value of r = 0.647 (considerable positive correlation)
was obtained with a significant p value (0.07), that is, the increase in mPAP is accompanied by an increase in PSAP; no previous studies of the relationship between mPAP and PSAP were found. The relationship between mPAP and VmxRT was
evaluated, finding a value of r = 0.612 (considerable positive correlation), a value of p = 0.012 was obtained, being statistically significant, that is, the increase in mPAP is accompanied by an increase in VmxRT; no previous studies
were found evaluating the relationship between mPAP and VmxRT. The relationship between mPAP and FDVD was evaluated, obtaining a value of r = 0.708 (considerable positive correlation), with a significant p value (0.02). No antecedents
were found in previous studies that have correlated mPAP with DDVD. In the study, the results are due to the existence of DDVD in the patients with CML and PAH studied. The relationship between the probability for PAH with mPAP was evaluated,
obtaining a value of r = 0.706 (considerable positive correlation), the p value obtained was significant (0.08); no study of this relationship was found in previous publications. This study result was obtained because there is a significant
relationship between the probability of PAH and the mPAP value in the patients studied, where the higher the mPAP value the greater the probability of PAH.
With the evaluation of the relationship between echocardiographic findings, a significant association was found between the estimation of mPAP (to define PAH by echocardiography when mPAP≥25 mmHg) and the echocardiographic findings
related to PAH (VmxRT, PSAP), which are obtained independently of mPAP; Furthermore, FDVD was evaluated, a parameter that is not usually evaluated routinely and that in this study was positively correlated with mPAP and the presence of
PAH. In addition, the estimate of mPAP was significantly related to the probability of PAH obtained by echocardiographic findings. No previous studies were found where a relationship of echocardiographic variables for PAH in CML patients
treated with dasatinib was performed. These study results are due to the fact that all echocardiographic findings are significantly related to the presence of PAH (determined by mPAP), where an echocardiographic estimate of mPAP less than
25 mmHg does not exclude the presence of PAH, and other evaluations must be performed. echocardiographic.
This study aims to support the Cardiology and Hematology Service, so that patients with CML treated with dasatinib undergo close monitoring with an echocardiogram prior to and during the start of treatment, evaluating all the echocardiographic
parameters that showed usefulness, due to a positive correlation with PAH estimated by mPAP, specifically when PAH cannot be ruled out only with an echocardiographic parameter and it is not feasible to perform an invasive study by right
catheterization. The aim is to introduce the routine evaluation of FDVD in patients treated with TKI since this finding appears early and translates into disease progression. Also, to enter the field of cardioncology to carry out early
cardiovascular diagnoses (by ECOTT), and to establish treatments aimed at heart failure and PAH in cancer patients who should be treated with some TKI.
Documenting PAH early in CML patients on dasatinib is important for making changes in treatment or taking action to limit cardiopulmonary damage.
The limitations of this research were the sample size, which was affected by being a retrospective study; Furthermore, since it is a correlational study, the results found do not indicate the existence of a cause-and-effect relationship
between the variables involved.
CONCLUSION
The relation between dasatinib treatment time and mPAP values had a medium negative correlation. The relation of mPAP with the dose of dasatinib had a mean positive correlation. Each of the following relationships mPAP with PSAP, mPAP with VmxRT, mPAP
with FDVD, and probability for HAP with mPAP had a considerable positive correlation.
The echocardiographic parameters mPAP, VmxRT, PSAP, DDVD, and echocardiographic probability for PAH are useful and necessary values to assess PAH. The determination of all these parameters should be carried out early and as a follow-up,
since a considerable positive relationship was found for each one with the presence of PAH, which is not dependent on the treatment time or the dose of dasatinib.
Authorship contributions: KPN, EHJ and PZM have participated in the conception, design of the article, analysis and interpretation of data, writing of the critical article of the article, approval of the final version, statistical
advice and technical or administrative advice. AJMJ and MRJS in data analysis and interpretation, writing of the article, critical review of the article, approval of the final version, statistical advice and technical or administrative
advice. AGG and DEHS in analysis and interpretation of data and contribution of patients or study material. KPN and DEHS in the collection of results, analysis and interpretation of data, writing of the article, approval of the
final version and contribution of patients or study material. EHJ in the conception and design of the article, analysis and interpretation of data, writing of the article, critical review of the article, review of the final version
and technical or administrative advice. AMGL in writing the article, critical review of the article and approval of the final version. KPN, AJMJ and AGG in writing the article, critical review of the article and approval of the
final version. All authors reviewed and approved the final version of the document.
Funding: This research has not received any specific grant from agencies in the public, commercial, or non-profit sectors.
Conflicts of interest: none
Received: May 12, 2021
Approved: July 17, 2021
Correspondence: María del Rayo Juárez Santiesteban
Address: Calle 2 norte # 2004. Colonia Centro. CP 72000. Puebla, Puebla-México
Phone: +521 2224476686
Email: mariravo@hotmail.com
REFERENCIAS BIBLIOGRÁFICAS
