CARTA AL EDITOR
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i2.4401
PERUVIAN EXPERIENCE ON THE HUMAN IMMUNODEFICIENCY VIRUS DIAGNOSTIC FLOWCHART
EXPERIENCIA PERUANA SOBRE EL FLUJOGRAMA DE DIAGNÓSTICO DEL VIRUS DE INMUNODEFICIENCIA HUMANA
Eduardo Miranda-Ulloa1,a, Soledad Romero-Ruiz1,b, Maribel Acuña1,b Ronal Briceño-Espinoza1,c, George Obregon1,d, Dilan Suárez-Agüero1,e
1 National Reference Laboratory Sexually Transmitted Virus HIV/AIDS, National Center for Public
Health, National Institute of Health. Lima-Peru.
a Biologist, Master in Microbiology
b Biologist
c Medical Technologist
d Biologist, PhD in Public Management and Governance
e Bachelor in Microbiology and Parasitology
Mr Editor:
The Joint United Nations Program on HIV/AIDS (UNAIDS) proposed as a goal that the countries
reach 95-95-95 by the year 2030, in other words, that 95% of people living with HIV (PLH) are diagnosed
and of these that 95% receive antiretroviral treatment (ART) and at least 95% have undetectable viral
load or viral suppression(1).
En el 2014 el Perú alcanzó la fórmula 64-46-36, por estas razones el Ministerio de Salud (MINSA) realiza
numerosos esfuerzos para cerrar brechas y lograr este objetivo, es por ello que con base en la
evidencia, periódicamente se modifican las fórmulas técnicas. estándares de salud para mejorar la
cobertura y el abordaje de las PLH. (2).
In order to close the first gap, that is, the first 95, the MINSA began expanding the coverage
of HIV diagnosis a few years ago through the use of rapid tests at all levels of care in the health
system, as This is why the World Health Organization has recommended modifications in the diagnostic
flowcharts, which shorten the time to define a person with HIV infection and that he or she can receive
early treatment. (3).
The HIV diagnosis flowcharts for people over 18 months of age, which are contained in the three
technical health standards currently in force, define a case of HIV infection as those people who have
two reactive results to two screening tests from different manufacturers. or of a different principle,
considering screening tests to be the Rapid Test (RT), Enzyme-Linked Immunosorbent Assay (ELISA),
Chemiluminescence (CLIA) and Electrochemiluminescence (ECLIA). It is important to highlight the
following cases as cases: a) two reactive results of two third-generation RPs from different
manufacturers b) two reactive results of two RPs, one third-generation and one fourth-generation c) One
third- or fourth-generation RP generation and an ELISA or equivalent with reactive results d) A third or
fourth generation RP with a reactive result and a positive confirmatory test: viral load, indirect
immunofluorescence (IIF) or immunoblot(4-6).
In the novelty of these flowcharts, the consideration of two reactive results to two screening
tests as a case of HIV infection is highlighted(4,5); however,
there is currently no relevant information on the experience of its applicability in the Peruvian
population. For these reasons, the following objective was set: Identify the results of confirmatory HIV
serological tests in Peruvian samples with two reactive results to two different screening tests.
An observational descriptive study was carried out during November 2021 at the National
Reference Laboratory for Sexually Transmitted Viruses HIV/AIDS of the National Institute of Health
(INS); Secondary data of diagnostic results were analyzed without access to patient identification, so
it did not require the approval of an ethics committee since it was a necessity in national HIV
surveillance to have information to provide technical guidance to the healthcare workers.
The search was carried out for the screening results recorded in the diagnostic files that were
sent by the different health establishments in Peru to the INS for the confirmation of HIV in the period
from January 1 to December 30, 2019. Likewise, the results of the confirmatory serological tests issued
by the INS were obtained from the work protocols and the NetLab 1 laboratory information system.
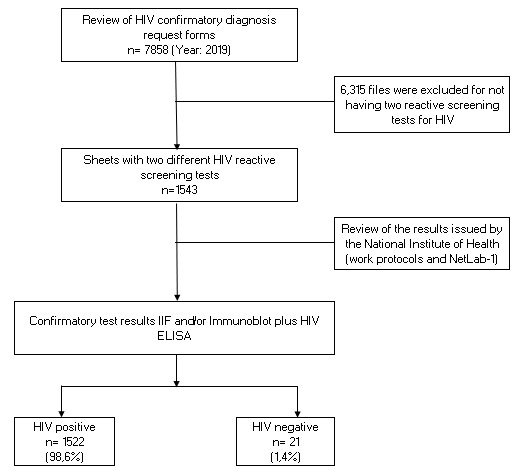
Among the results, it was determined that of a total of 7,858 samples received, 1,543 had two
reactive results to two different screening tests (age group: from eighteen months to thirteen years =
12; from fourteen to seventeen years = 39; years = 1492) at the same time it is evident among the main
findings that 98.6% (1522/1543) had a concordant positive result by means of the confirmatory
serological tests (IFI and Immunoblot) (See Figure N°1)
It is important to highlight that only 1.4% (21/1543) had negative results for HIV, verifying that this
small percentage that had the condition of defined case for HIV, showed to be false reagents. However,
it is important to highlight that the diagnostic flowchart indicates that all cases with two reactive
screening test results should immediately undergo the HIV viral load test (test considered confirmatory
and baseline for the start of monitoring) before starting ART(4,5). This fraction of cases could be identified as undetectable by the viral
load test (viral RNA), so only these few samples should be referred to for serological confirmation,
because there would be a risk of administering ART to patients who actually are HIV negative.
It should be noted that there are reports that HIV screening tests can generate false reactive
results in hemolyzed, lipemic or contaminated samples, also in people with anti-HLA antibodies,
neoplasms, autoimmune diseases and in multiparous women(3).
The high concordance demonstrated in the studied samples that were positive to the confirmatory
serological tests (98.6%) allows us to show that the HIV diagnosis flowchart in the Peruvian population
presents a high reliability in its application, consequently the results obtained allow us to indicate
that samples with two reactive results to screening tests should not be referred for serological
confirmation (IIF and Immunoblot) and should be sent directly for viral load analysis; in this way,
patients would access timely ART.
This experience allows us to conclude that the HIV diagnosis flowchart in people older than 18
months works with high reliability in Peru.
Authorship contributions: The authors participated in the conception and design of the
study; collection, analysis and interpretation of the results; drafting, critical review and
approval of the final version of the letter.
Funding sources: The study was carried out within the framework of the regular activities
of the National Institute of Health (national surveillance of HIV).
Conflicts of Interest: The authors declare no conflict of interest.
Received: December 10, 2021
Approved: December 22, 2021
Correspondence: Eduardo Miranda Ulloa
Address: Defensores del Morro 2268, Chorrillos, Peru.
Telephone number: (+51) 977783088
E-mail: fernandoul@hotmail.com
REFERENCES