EDITORIAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i2.4786
HEMATOPOIETIC STEM CELL TRANSPLANTATION IN PERU: EXPERIENCE AND CHALLENGES OF THE LARGEST TRANSPLANTATION CENTER IN PERU
TRASPLANTE DE CÉLULAS MADRE HEMATOPOYÉTICAS EN EL PERÚ: EXPERIENCIA Y DESAFÍOS DEL CENTRO DE TRASPLANTE MÁS GRANDE DEL PERÚ
Lourdes Aranda-Gomero1, Rafael Pichardo-Rodriguez2, Ivan Fernandez-Vertíz1, Alfredo Wong-Chang1,2
1 Unidad de trasplante de Médula Ósea. Hospital Nacional Edgardo Rebagliati Martins, Lima-Perú.
2 Instituto de Investigaciones en Ciencias Biomédicas (INICIB). Universidad Ricardo Palma,
Lima-Perú.
HEMATOPOIETIC STEM TRANSPLANTATION IN LATIN AMERICA AND PERU
The rst transplants performed in Latin America began in the 1980s, and the number has been increasing
(5) continuously since . In Peru, starting in 1994, the rst transplants began
to be performed. Since that date, the bone marrow transplant unit (UTMO) of the "Edgardo Rebagliati
Martins" National Hospital (HNERM) as well as a few other Peruvians centers, has been carrying out HPT
in its different modalities: Autologous transplants in which the same patient is the donor of their
cells, Compatible Allogeneic Transplantation in which a consanguineous sibling donates the cells as they
are 100% compatible according to their HLA and nally from the 2015 a new unique methodology in the
country that allows transplants with direct relatives of the same family nucleus (Parents and Siblings)
who only present 50% of the compatibility also known as Haploidentical (1,6). This experience is demonstrated with more than 1500 transplants performed.
Currently, by the year 2022, at the national level, there were only seven health establishments
accredited by the Ministry of Health to carry out TPH7. Only 4 of these centers are authorized to
perform allogeneic transplants, which (7) are the priority in pediatric
patients with leukemia . The HNERM UTMO service has contributed to more than half of all transplants
performed nationwide, which by 2020 were 155 procedures.
PROFILE OF THE PATIENTS UNDERGOING HSCT
Among the characteristics of the patients undergoing HSCT in our unit, the most frequent gender in general for all transplants was male, aged between 18 and 51 years. Table 1 summarizes the proles of patients undergoing HSCT at the UTMO of the HNERM.
SURVIVAL OF TRANSPLANTED PATIENTS IN THE UTMO
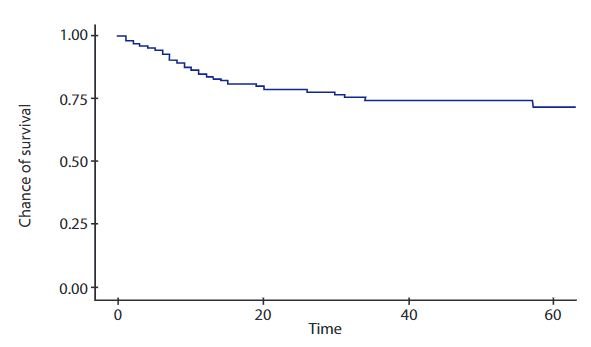
It has been possible to achieve an overall survival at 60 months (5 years) in our patients of 75% for allogeneic TPH and up to 55% for haploidentical TPH. Figures 1 and 2 show the survival curves estimated by Kaplan-Meier. On the other hand, the overall survival for patients undergoing autologous HPCT at 5 years is only 50%.
IMPACT OF THE COVID-19 PANDEMIC ON THE TPH PROGRAM
The pandemic caused by coronavirus disease 2019, better known as COVID-19, which was declared in March
2020, has generated a global health crisis, with a significant impact on bone marrow transplant units
around the world(8).
The UTMO of the HNERM had a negative impact, reducing by 40% the number of transplants performed
during the year 2020 compared to the previous year. It found an incidence of 13.5%, with mild cases
being the most frequent group (83%) followed by severe cases (17%) and lethality of 8%, three times
above the average Peruvian population. It was also found that only 25% required specific treatment for
symptoms.
PERSPECTIVES FOR THE FUTURE
Since its creation in 1994, the UTMO of the HNERM has been making continuous efforts to provide a warm
service with the highest quality standards to patients with diseases that compromise the functioning of
the bone marrow, offering an opportunity for treatment and in some cases, even the cure of highly
aggressive pathologies(1).
Despite the progressive increase in procedures performed at the national level, access to bone
marrow transplantation is still insufficient, so greater efforts are required to close this
infrastructure gap.
In this year, after the ravages caused by the pandemic, we want to be able to continue growing
as a unit and implementing new technologies and transplant platforms such as the unrelated, the
promotion and implementation of a scientific research unit that allows specific analyzes of our
population, such as the evaluation of the quality of life after transplantation, as well as the
development of cell therapy that allows us to improve our transplant processes and obtain the best
possible results for the sake of patient health. For this reason, we thank all our patients for the
trust placed in the HNERM UTMO to treat their diseases.
Table 1. Prole of patients undergoing HSCT in the UTMO of the HNERM
| Allogeneic | Haploidentical | Autologous | |
|---|---|---|---|
| Age | 30 years | 18.5 years | 51 years |
| Gender | Man | Man | Man |
| Diagnosis | B-ALL | B-ALL* | Multiple myeloma |
| Donor | Brother | Father | Doesn’t apply / Does not apply |
| Blood group | O+ | O+ | O+ |
| *Leucemia linfoblástica aguda B | |||
Correspondence: Rafael Pichardo-Rodriguez
Address: Av Brasil 748, Jesus María,Lima-Perú
Telephone number: 989370702
E-mail: rafael_martin1352@hotmail.com
REFERENCES