ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i3.4951
PREVALENCE AND FACTORS ASSOCIATED WITH RETINOPATHY IN PATIENTS OF THE INTEGRAL DIABETES PROGRAM OF THE SAN GENARO DE VILLA CHORILLOS HEALTH CENTER, LIMA-PERU
PREVALENCIA DE RETINOPATIA EN PACIENTES DEL PROGRAMA INTEGRAL DE DIABETES DEL CENTRO DE SALUD SAN GENARO DE VILLA, CHORILLOS, LIMA-PERÚ
John Longa-López1,a, Miguel Mavila-Salon2,b, Luis Rodriguez-Dominguez2,b
1CS San Genaro de Villa- Chorrillos-Lima-Perú
2CMI Daniel Alcides Carrión -San Juan de Miraflores-Lima-Perú.
aMedical Endocrinologist, Mg in Public Health
bOphthalmologist
ABSTRACT
Objectives: To determine the prevalence and factors associated with retinopathy in patients of the Comprehensive Diabetes Program of the San Genaro Health Center in Villa Chorrillos. Methods: Descriptive, observational, cross-sectional, prospective study; with a sample of 119 adults and older adults. Non-probabilistic convenience sampling was used. The variables studied were diabetic retinopathy, type of diabetic retinopathy, degree of diabetic retinopathy, age, sex, educational level, time of illness, time belonging to the program, type of treatment, personal history of arterial hypertension, personal history of dyslipidemia, mean systolic blood pressure (SBP), mean diastolic blood pressure (DBP), Body Mass Index (BMI), Glycosylated Hemoglobin (HbA1c), Total Cholesterol, LDL Cholesterol, HDL Cholesterol, Triglycerides, creatinine clearance, microalbuminuria, visual efficiency of Snell-Sterling, associated ocular pathology and ocular pressure. Descriptive statistical methods were used. Results: The prevalence of diabetic retinopathy (DR) was 16%, of which 73.7% is non-proliferative DR, 21.2% proliferative DR, and 5.2% macular edema, and in relation to the degrees of Non-Proliferative DR 60% is mild, 33.3% moderate and 6.7% severe and in Proliferative DR 33.3% is early, 33.3% high risk and 33. 3% Severe. The biochemical value that showed a considerable difference was microalbuminuria, reaching a value of 356.93 mg/dl/24hrs. Conclusions: The prevalence of retinopathy is 16%, of which 73.7% is nonproliferative retinopathy, 21.2% proliferative retinopathy, and 5.2% macular edema.
Keywords: Prevalence; diabetic retinopathy; diabetes mellitus. (source: DeCS BIREME)
RESUMEN
Objetivos: Determinar la prevalencia y los factores asociados a retinopatía en pacientes del Programa Integral de Diabetes del Centro de Salud San Genaro de Villa Chorrillos. Métodos: Estudio descriptivo, observacional, transversal, prospectivo; con una muestra de 119 adultos y adultos mayores. Se utilizo el muestreo no probabilístico por conveniencia. Las variables estudiadas fueron retinopatía diabética, Tipo de retinopatía diabética, grado de retinopatía diabética, edad, sexo, grado de instrucción, tiempo de enfermedad, tiempo de pertenencia la programa, tipo de tratamiento, antecedente personal de hipertensión arterial, antecedente personal de dislipidemia, presión arterial sistólica (PAS) promedio, presión arterial diastólica (PAD) promedio, Índice de masa corporal (IMC), Hemoglobina Glicosilada (HbA1c), Colesterol total, Colesterol LDL, Colesterol HDL, Triglicéridos, depuración de creatinina, microalbuminuria, eficiencia visual de Snell-Sterlling, patología ocular asociada y presión ocular. Se emplearon métodos estadísticos descriptivos. Resultados: La prevalencia de retinopatía diabética (RD) fue de 15,1% de los cuales el 77,8% es RD No proliferativa y el 22,2% RD proliferativa. En relación a los grados en la RD No Proliferativa el 64,3% es leve y el 35,7% moderada; y en la RD Proliferativa el 25% es temprana, el 25% de alto riesgo y el 50% severa. El valor bioquímico que mostro una considerable diferencia fue la microalbuminuria alcanzando un valor de 356,9 mg/dl/24hrs. Conclusiones: La prevalencia de retinopatía es de 15,1% de los cuales el 77,8%% es retinopatía no proliferativa y de 22,2% retinopatía proliferativa y los factores asociados fueron la presión arterial sistólica (p<0,001) y la microalbuminuria (p<0,001).
Palabras Clave: Prevalencia; Retinopatía diabética; Diabetes mellitus. (fuente: DeCS BIREME).
INTRODUCTION
Diabetic retinopathy (DR) is the leading cause of new cases of legal blindness among adults 20 to 74 years of age in the United States (1). Vision loss due to diabetic retinopathy occurs through a variety of mechanisms, including retinal detachment, vitreous or premacular hemorrhage, associated neovascular glaucoma, and macular edema (2). The presence of retinopathy can indicate a dysfunction in the microcirculation of other organs and systems (3,4). Therefore, investigating the prevalence of diabetic retinopathy is important since it constitutes a key indicator of systemic microvascular complications and a sentinel indicator of the incidence of diabetes.
In our country, according to the epidemiological surveillance system of Diabetes 2021 of the National Center for Epidemiology, prevention, and control of diseases of 96 hospitals, 62 health centers, 153 health posts, and 2 reporting polyclinics, only 26.31% had results of the evaluation of macro and microvascular complications, 6.94% corresponding to diabetic retinopathy. As we can see, there is an under-evaluation and under-reporting of one of the complications with the highest impact on the quality of life of patients with diabetes mellitus.
It is important to mention that showing the presence of retinopathy among the first causes of outpatient consultation shows us an indication of the repercussions of the epidemiological transition in the country, that is, the increase in non-communicable diseases to communicable ones.
The prevalence of Diabetic Retinopathy increases with the duration of diabetes. In the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), the prevalence of retinopathy in patients with early onset was 8% at 3 years, 25% at 5 years, 60% at 10 years, and 80% at 15 years.
At the Wilmer Ophthalmological Institute of the University, Johns Hopkins in Baltimore, it was shown that the cost to avoid blindness due to proliferative DR in insulin-dependent patients would be 966 dollars per person per year and to preserve central vision due to macular edema would be 1118 dollars annual. This would represent one-seventh of the average cost of a year of social security for the visually impaired.(5) The current evidence shows that the increase in health costs is associated with the progression of retinopathy (6) and cost-effectiveness studies tip the balance in favor of annual screening for diabetic retinopathy and subsequent treatment in type 1 and 2 diabetics compared with no screening. (7,8) If all patients with type 2 diabetes receive the recommended treatment, the net projected savings are greater than $472.1 million and 94,304 vision-related person-years. Including each additional person with type 2 diabetes in current recommended eye care results in an average net savings of $975 per person, even if all care costs are borne by the federal government.(9)
The vast majority of patients who develop diabetic retinopathy do not present symptoms until very late stages (by which time it may be too late for effective treatment). There is evidence that retinopathy begins to develop 7 years before the clinical diagnosis of type 2 diabetes mellitus (10). Because the rate of progression may be rapid, and therapy may be beneficial in both improving symptoms and reducing the rate of disease progression, periodic evaluation of patients with diabetes mellitus is important to prevent retinal disease development.
In conclusion, diabetic retinopathy constitutes a nosological entity whose resizing and clinical-epidemiological characterization is transcendental due to its economic, social, etc., impact. that it involves, but also because of the implications that its identification entails in decision-making in public health, especially in scenarios such as the health jurisdiction of the San Genaro de Villa Health Center, a primary care facility that covers a diabetic population in whose A set of health determinants come together, whose dynamic interaction is essential to elucidate in order to be able to implement prevention strategies at all levels.
MÉTODOS
Design and Study Area
A descriptive, observational, cross-sectional study was conducted.
The investigation was carried out in the San Genaro de Villa health center located in the District of Chorrillos, category I-3 where preventive-promotional and assistance activities are carried out. In November 2005, the Comprehensive Diabetes Program of the San Genaro de Villa Health Center, a program that was born as a local strategy within the Framework of the Comprehensive Health Care Model and that at the date of the study has 120 patients, who have been progressively enrolled within a comprehensive and holistic management strategy, before the COVID 19 pandemic.
Population and Sample
The study population was made up of all adults and older adults of both sexes diagnosed with type 2 diabetes mellitus, belonging to the Comprehensive Diabetes Program of the San Genaro de Villa Health Center during the period of the present investigation, which amounted to 120. The sampling for the present investigation was of a non-probabilistic type for convenience since all the patients who met the inclusion and exclusion criteria were included, these 119 patients.
Variables and Instrument
The variables that were studied were:
Diabetic Retinopathy, Inflammatory disease of the retina related to diabetes.
Types of Diabetic Retinopathy: Characterization of retinopathy according to clinical findings and according to previously established criteria.
Grades of Diabetic Retinopathy: Determination of the degree of severity of retinal damage for prognostic and therapeutic purposes.
Sex: Characteristics of individuals who identify as male or female.
Age: Is defined as the number of years, months, and days completed on the instrument's application date.
Level of education: Degree of the academic level reached.
Time of illness: Years elapsed from the medical diagnosis of diabetes mellitus to the date of the interview.
Time of belonging to the program: Years elapsed from enrollment in the Program to the date of the interview.
Type of Treatment: It is the name of the medication prescribed and consumed for therapeutic purposes.
Personal History of AHT: It is the prior knowledge by the patient of suffering from a disease.
Personal History of Dyslipidemia: It is the prior knowledge by the patient of suffering from a disease.
Systolic Blood Pressure (SBP): It is the maximum value of blood pressure in systole.
Diastolic Blood Pressure (DBP): It is the minimum value of blood pressure when the heart is in diastole.
Body Mass Index (BMI): It is the measure of association between weight and height of a person to assess their nutritional status.
Glycosylated Hemoglobin: Heteroprotein of the blood that results from the union of Hb with free carbohydrates attached to carbon chains with acid functions in carbon 3 and 4. It is used to evaluate the degree of metabolic control of diabetes in the last 3-4 months.
Total Cholesterol: It is the cholesterol present in the plasmatic lipoproteins that are in the fasting state; that is, it represents the cholesterol carried by the lipoproteins: HDL, VLDL, and LDL.
LDL Cholesterol: Low-density lipoproteins with atherogenic capacity.
HDL Cholesterol: It is the cholesterol found only in the HDL lipoprotein. It evaluates the body's ability to remove excess cholesterol from the periphery. Triglycerides are mainly represented in the VLDL lipoprotein because, in basal conditions, there are no chylomicrons, and HDL and LDL carry very little TG. This test evaluates the hepatic synthesis of VLDL.
Creatinine Clearance: Allows the evaluation of the glomerular filtration rate by relating the determination of urinary and serum creatinine.
Microalbuminuria: Is the urinary excretion of albumin and is a marker of early kidney disease.
Visual Efficiency: The degree of efficiency of the vision to perceive, detect or identify spatial objects with good lighting conditions.
Associated Ocular Pathology: :Diseases that compromise the different structures of the eye.
Ocular Pressure: :The pressure exerted by the ocular fluids against the wall of the eye is necessary for this organ to remain distended.
Procedure
In the data collection form, the information on the variables: level of education, time of illness, time belonging to the program, type of treatment, personal history of arterial hypertension and personal history of dyslipidemia were obtained through the interview technique. ; The date of birth (which was used to calculate the patient's chronological age) and sex were recorded from the national identity document (DNI) of the interviewee. On the other hand, the variables: systolic blood pressure (SBP), diastolic blood pressure (DBP), weight, and height were obtained by direct measurement, being the body mass index (BMI) calculated from the data recorded in the weight and height of the patient. patient. Regarding the variables: glycosylated hemoglobin (HbA1c), total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, creatinine clearance, and microalbuminuria, the corresponding biochemical laboratory analyzes were recorded. Regarding the variables: visual acuity and ocular pressure were obtained from the clinical evaluation carried out by an ophthalmologist who, in turn, recorded the associated ocular pathology. To the visual efficiency variable, was obtained through the Snell Sterling visual acuity equivalencies. Finally, for the variables: prevalence of retinopathy, type of retinopathy, and degrees of retinopathy, the corresponding fluorescein angiography evaluated by an ophthalmologist with a retina subspecialty was recorded.
Statistical Analysis
The data obtained in the present investigation were processed using the statistical package PASW version 18.0. For the analysis of the variables, descriptive statistics were used, being in the case of quantitative variables, the measures of central tendency used, and in the case of categorical variables, the distribution of frequencies.
Ethical Aspects
Approval was obtained from the ethics committee of the Postgraduate School of the Federico Villarreal National University
RESULTS
From the graph, it can be seen that of the total number of cases of Proliferative Diabetic Retinopathy, 33.3% correspond to the Early degree, 33.3% to the high-risk degree and 33.3% to the severe degree.
Table 1. Prevalence of retinopathy according to clinical, epidemiological characteristics of the Comprehensive Diabetes Program of CS San Genaro de Villa, Chorrillos
| With Diabetic Retinopathy | Without Diabetic Retinopathy | |
|---|---|---|
| Average age | 62.68 years | 58.30 years |
| Sex | ||
| Male | 26.3% (5) | 17% (17) |
| Female | 73.7 % (14) | 83% (83) |
| Level of Instruction | ||
| None | 15.8% (3) | 16% (16) |
| Primary | 36.8% (7) | 42% (42) |
| Secondary | 42.1% (8) | 36% (36) |
| Techn. Superior | 0% | 5% (5) |
| University | 5.3% (1) | 1%(1) |
| Time of illness | 7.87 years | 6.97 years |
| Time belonging to the program | 2.53 years | 2.15 years |
| Type of Treatment | ||
| ADO | 89.5% (17) | 90%(90) |
| Insulin | 0% | 2%(2) |
| ADO + Insulin | 10.5% (2) | 8% (8) |
| History of Arterial Hypertension | ||
| Yes | 52.6% (10) | 26.3%(26) |
| No | 47.4% (9) | 73.7% (73) |
| History of Dyslipidemia | ||
| Yes | 57.9% (11) | 48.5%(48) |
| No | 42.1% (18) | 51.5%(51) |
| Blood Pressure | ||
| Systolic | 154.47mmHg | 133.94mmHg |
| Diastolic | 74.21mmHg | 69.82mmHg |
| BMI | 27.72 kg/m2 | 29.30 kg/m2 |
| HbAc1 8.42 | 8.44 | % |
| Cholesterol7Total | 197.57 mg/dl | 199.02 mg/dl |
| LDL | 125.07 mg/dl | 125.02 mg/dl |
| HDL | 39.81 mg/dl | 38.52 mg/dl |
| Triglycerides | 176.00 mg/dl | 187.83 mg/dl |
| Creatinine clearance | 90.36 ml/min | 112.13 ml/min |
| Microalbuminuria | 35 mg/dl/24h | 36.74 mg/dl/24h |
| Visual Efficiency | ||
| Right Eye | 79.78% | 82.55% |
| Left Eye | 73.52% | 89.40% |
| Ocular Pressure | ||
| Right Eye | 15.56mmHg | 15.94mmHg |
| Left Eye | 15.78mmHg | 16.45mmHg |
| Associated ocular pathology | ||
| Cataract | 52.6% (10) | 25% ( 25) |
| Glaucoma | 0% | 10% (10) |
| C Atarata + Glaucoma | 5.3% (1) | 6% (6) |
| Others/none | 42.1% (8) | 59% (59) |
In this table, the prevalence of Diabetic Retinopathy can be observed according to the clinical epidemiological characteristics studied in this investigation
DISCUSSION
In this investigation, the determination of the prevalence of diabetic retinopathy in the group studied was 16% , this figure is lower than that reported by the National Health and Nutrition Survey III (NHANES III) from 2005 to 2008 in the United States in the 28.5% of retinopathy is reported in the diabetic population.
On the other hand, the literature refers to the influence of some genetic and ethnic factors in the pathophysiology and natural history of diabetic retinopathy. These factors could explain the epidemiological variability in the frequency of presentation of said event. However, studies carried out in the Peruvian population, such as that of Juan Amaral et al. (11), who evaluated 849 patients from the Social Security Diabetes Mellitus Program in the city of Piura, found a prevalence of 30%. This figure does not differ much from the one found by Teresa Mendizábal et al. (12) who evaluated 48 patients from the Endocrinology Service of the Daniel Alcides Carrión Hospital in the city of Lima, finding a 26% prevalence of diabetic retinopathy. These figures reported at the national level are even higher than the one found in the present investigation, which can be explained due to the sample size of the different investigations referred to, as well as the factor related to the type of population included in the present investigations and the methodology used in the diagnosis which makes statistical comparisons difficult.
Concerning the prevalence of the different types of diabetic retinopathy, as we can see, Non-Proliferative Retinopathy is the most frequent with 73.7% followed by Proliferative with 21.1% and 5.2% presenting with Edema. Macular.
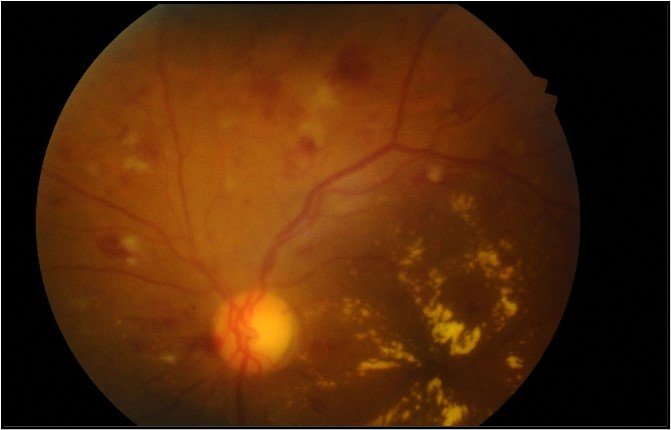
Pink ANOON, small papillary excavation, applied retina, decreased macular brightness with retinal thickening and exudates affecting the entire macula, microaneurysms distributed in four quadrants.
These figures coincide with what was reported by Juan Amaral Luna et al. (11) in the Piuran population of our country, which refers to a prevalence of Non-Proliferative Retinopathy of 81% as well as 19% of Proliferative Retinopathy, however, they report a higher proportion of Macular edema of the order of 35%, much higher than the figure found by our research. Feng Wua Huang et al. (13) in the Chinese population coincidentally found a prevalence of Macular Edema of 5.2%, equal to that reported in our research.
When we look at the prevalence of Diabetic retinopathy according to age, we can see that the average age of patients with diabetic retinopathy is greater than that of those who do not present the microvascular complication, being able to observe that the age group of older adults has a higher proportion of cases of retinopathy than the group of adults who belong to the Comprehensive Diabetes Program of CS San Genaro de Villa. These frequency data are related to what was found by Afra A Al-Sarraf et al. (14) , who in their study “Prevalence and Factors Associated with Diabetic Retinopathy, a Multi-centric Study in Kuwait” reports that of the personal factors examined, age was the only significant determinant of diabetic retinopathy (OR= 2.2, 95% CI: 1.1-5.2) as well as the age groups 50 to 59 and > 60 years compared with those under 40 years of age (OR= 4.6, 95% CI 2.0-11.0). This higher frequency of diabetic retinopathy in older adults could be related to the longer time living with the underlying disease and, therefore, greater exposure to chronic hyperglycemia, a preponderant pathophysiological factor in the appearance of said microvascular complication.
When the prevalence of retinopathy is described according to the time of illness, the average time of illness of patients with diabetic retinopathy is slightly higher than that of patients who do not present the complication. In relation to the time of illness, various studies such as the NHANES III Survey find that this factor is independently associated with the presence of retinopathy (OR: 1.06 per year of duration, CI 95%: 1.03-1.10), data that is corroborated by Afra A Al-Sarraf et al. (14) who found that the duration of diabetes is a significant predictor of diabetic retinopathy (OR=2.6, 95% CI: 1.61-4.2).
Studies such as the Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS) have shown that chronic hyperglycemia is a preponderant factor in the induction of retinal lesions, from which we can infer that a longer duration of disease would be associated with a greater probability of developing long-standing hyperglycemia and subsequent retinal lesions.
When we describe the prevalence of diabetic retinopathy according to the sex of the patients evaluated, we can see that although it is true that the greater proportion of patients with retinopathy is women (73.7%) compared to 26.3% of men who have the complication, we must take into account that in the Comprehensive Diabetes Program of the CS San Genaro de Villa the highest proportion of patients who attend it are women (81.7%) compared to 18.3% of men. For this reason, when we analyze by gender, we find that the proportion of male patients with retinopathy (22.72%) is greater than the proportion of female patients with said complication (14.43%). This is related to what was found in the NHANES III from 2005 to 2008, which found that diabetic retinopathy is slightly more frequent in men than in women with diabetes, 31.6% (95% CI: 26.8% -36 .8%) versus 25.7% (95% CI: 21.7% -30.1%, p = 0.04). Likewise, according to NHANES III, the male sex was independently associated with diabetic retinopathy (odds ratio [OR] = 2.07, 95% CI: 1.39-3.10).
When we describe the prevalence of retinopathy according to the type of treatment, we can see that the majority of patients with retinopathy use oral antidiabetics (89.5%) compared to 10.5% of patients with the complication who already use insulin. either alone or in combination with oral antidiabetics. If we analyze by treatment groups the total number of patients who receive only Oral antidiabetics, 15.88% have Diabetic Retinopathy, and in the case of patients who receive oral antidiabetic therapy plus insulin, 20% have Diabetic Retinopathy. This slight numerical superiority in the prevalence of retinopathy in insulin-dependent patients is related to what was found in other investigations, such as the NHANES III from 2005 to 2008, which reported that the use of insulin was independently associated with the presence of retinopathy (OR, 3.23, 95% CI: 1.99 to 5.26). Researchers such as Afra A Al-Sarraf et al. (14), also found that patients with diabetes mellitus treated with insulin were more likely to develop diabetic retinopathy (OR=8, 95% CI: 3.5-19.4. At, Juan Amaral Luna et al.(11) found that the type of treatment was a factor related to the appearance of diabetic retinopathy.
When we analyzed the prevalence of diabetic retinopathy according to total cholesterol, LDL-C, HDL-C or triglycerides, we did not observe a numerically important difference in both groups with and without retinopathy, this could be since many of the patients of the Comprehensive Diabetes Program of CS San Genaro de Villa who present with dyslipidemia receive lipid-lowering treatment to treat said alteration of lipid metabolism, which could make the numerical difference to which we refer imperceptibly.This would be related to the findings found when we evaluated the variable prevalence of diabetic retinopathy according to the history of dyslipidemia in which we can rather appreciate that the highest proportion (57.%) of patients who have retinopathy have a history of dyslipidemia while on the other hand, of patients who do not have retinopathy the largest proportion (51.5%) do not have the history.
When we evaluate the prevalence of retinopathy according to values of systolic blood pressure (SBP), and diastolic blood pressure (DBP) in the patients studied, we find that the average SBP in patients with retinopathy is 154.474 mmHg, while in patients who do not have the complication the average is 133.940 mmHg, to DBP the averages are respectively: 74.211 mmHg and 69.820 mmHg. In both cases, whether SBP or DBP, patients with diabetic retinopathy have a higher average blood pressure than those who do not have the complication; These descriptive data are related to the analytical findings of studies such as the NHANES III, in which SBP was found to be independently associated with the presence of diabetic retinopathy (OR, 1.03 per mm Hg; 95% CI: 1.02-1.03). . Concerning blood pressure and its impact on the induction of retinal lesions, current evidence supports that control of arterial hypertension reduces the rate of progression of diabetic retinopathy and reduces the risk of vitreous hemorrhage. Studies such as the United Kingdom Prospective Diabetes Study (UKPDS) found that after 8-9 years of intervention, the group of patients with lower blood pressure had a 24% reduction in diabetes-related endpoints and a 34% and 47% reduction in significantly impaired retinopathy and visual acuity, respectively. The improvement was manifested in all aspects: microaneurysms, hard exudates, cotton wool exudates, progression of retinopathy, need for photocoagulation, and blindness. Hence the importance of control. a comprehensive study of blood pressure in this group of patients.
The prevalence of diabetic retinopathy according to Body Mass Index (BMI) in patients with and without retinopathy, a slightly higher average BMI was found in patients who do not have the complication; these data differ from those found by Teresa Mendizábal et al.(12) in 2010, who after evaluating 48 patients with diabetes mellitus found that the average BMI at the time of evaluation was 29.5 and 25.9 for patients with and without microangiopathy, respectively. In this last study, as we can see, the patients with retinopathy had a higher BMI than those without the complication, which is the opposite of that found in the present investigation. This could be explained by the sample size included in the investigation by Mendizábal et al., which only included 48 diabetic patients. On the other hand, we must take into account that it is likely that patients who already have micro or macrovascular complications and who are included in a comprehensive program that reinforces the promotional, and preventive component, further intensify aspects such as nutrition and physical activity with the consequent impact on clinical anthropometric measurements and although obesity is associated with retinopathy, it is not clear if it is an independent factor or if it acts through other factors, such as the increased prevalence of arterial hypertension, hyperlipidemia or worse metabolic control(15-16).
When we describe the study variable prevalence of diabetic retinopathy according to glycosylated hemoglobin (HbA1c), we can see that the average value in patients with and without retinopathy is practically the same (8.4489 vs 8.7283). Again, surveys such as the NHANES III found that diabetic retinopathy was independently associated with a higher level of glycosylated hemoglobin (OR, 1.45; 95% CI: 1.20-1.75). Afra A Al-Sarraf et al. (14)also found that poor glycemic control were associated with the appearance of retinal lesions in diabetic patients (OR=2.0, 95% CI: 1.2-2.8). The UKPDS study(17) found that a 0.9% reduction in HbA1c over 10 years of follow-up in patients with newly diagnosed type II diabetes mellitus decreased the onset and progression of retinopathy by 25%. At 6 years of follow-up of 1,216 patients who did not have retinopathy at diagnosis, 22% developed some type of lesion, and of 703 who did at baseline, 29% worsened. The incidence and progression were related to the degree of control(18).
One way to assess kidney function and compromise in diabetic patients is by determining creatinine clearance and quantifying microscopic albumin loss called microalbuminuria. Precisely, evaluating these laboratory parameters allows us to identify other microvascular complications of the diabetic patient, such as nephropathy. In our study, we can appreciate the Prevalence of Diabetic Retinopathy according to Creatinine Clearance that the average of this parameter in patients with retinopathy is lower than patients without the complication (90.3653 ml/min vs 112.1364 ml/min respectively), which implicitly reflects a greater deterioration of renal function in the first group of patients. On the other hand, the Prevalence of Diabetic Retinopathy according to Microalbuminuria, the average of this parameter in patients with retinopathy is higher than those who do not have the complication (356.93 mg/dl/24 hours vs 36.7440 mg/dl/24 hours) already reflecting renal compromise in the former. These descriptive data are related to the findings of Afra A Al-Sarraf et al. (14), who found that nephropathy was significantly associated with diabetic retinopathy. The association between the development of diabetic retinopathy and nephropathy, independent of the degree of hyperglycemia and the duration of diabetes, suggests that common pathogenic factors may underlie the development of both complications.
When we evaluate the prevalence of diabetic retinopathy according to associated ocular pathology, we can see that cataract was the most frequent pathology in patients with retinopathy, with a 52.6% prevalence in this group. However, when we sub-analyze by type of pathology in both groups of patients with and without retinopathy, we see that of the total number of patients with cataracts, 71.4% do not have retinopathy. Various population studies have shown that the risk of cataracts is greater in the diabetic population. In a study(19) conducted in an Australian population, diabetes mellitus (DM) involved a relative risk of posterior subcapsular cataracts of 1.7 to 5.1.
Snell Sterling's visual efficiency is a way of expressing a person's degree of visual engagement as a percentage. As we can see from the Prevalence of Diabetic Retinopathy according to Right Eye Visual Efficiency and Prevalence of Diabetic Retinopathy according to Left Eye Visual Efficiency, the average visual efficiency calculated in both eyes is higher in patients without retinopathy than in those with microangiopathic complications. which is a logical consequence of less retinal compromise and, therefore, better visual acuity in the former. Precisely, Manuel E. Licea Puig et al. (20), after evaluating 110 diabetic patients with retinopathy, found 65.9% of them with visual efficiency below 84%, a value that when contrasted with our retinopathic population rises to 100%, that is, in our study all patients with retinopathy have a visual efficiency below the value found by Licea. Let us remember that the decrease in visual acuity that cannot be corrected with refraction constitutes one of the main clinical manifestations of diabetic patients with retinopathy.
If we evaluate the prevalence of diabetic retinopathy according to bilateral ocular pressure in both groups of patients with and without retinopathy, we will see, according to our results, that the average ocular pressure is practically similar (15.5632 mmHg and 15.9910 in the right eye; 15 .7842 and 16.4590 mmHg in the left eye, for patients with and without retinopathy, respectively). There is no agreement on whether DM poses an increased risk of glaucoma. Some studies observe twice the risk among the population with DM (21). However, others have not identified this relationship(22,23) .
ACKNOWLEDGMENTS
To the patients participating in the Comprehensive Diabetes Program for their active participation.
Contribuciones de Autoría: None
Financement: Self financed
Conflicts of interest: Los autores declaran no tener conflicto de interés.
Received: February 10, 2022
Approved: May 12, 2022
Correspondence: John Carlos M. Longa López
Address: Calle doña nelly 566 dpto 401- urb. santa rosa de surco- 2da etapa - surco
Telephone: 959912710
E-mail: johnlonga@gmail.com
Artículo publicado por la Revista de la Facultad de Medicina Humana de la Universidad Ricardo Palma. Es un articulo de acceso abierto, distribuido bajo los términos de la Licencia Creatvie Commons: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), que permite el uso no comercial, distribucion y reproducción en cualquier medio, siempre que la obra original sea debidamente citada. Para uso comercial, por favor póngase en contacto con revista.medicina@urp.edu.pe.
REFERENCES