ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i4.4995
CLINICAL, BIOCHEMICAL, ENDOSCOPIC, AND HISTOPATHOLOGICAL EVALUATION OF THE BIOLOGICAL TREATMENT OF ULCERATIVE COLITIS
EVALUACIÓN CLÍNICA, BIOQUÍMICA, ENDOSCÓPICA E HISTOPATOLÓGICA DEL TRATAMIENTO BIOLÓGICO DE COLITIS ULCERATIVA
Carlos Alberto López-Bernal1,a, Arturo García-Galicia1,b,f, Francisco Javier Quezada-Juárez2,c, Moisés Freddy Rojas-Illanes3,a, Briseida Rubio-Martínez4,a,Álvaro José Montiel-Jarquín1,c,f, Ingrid Jiménez-Luna1,d, Jorge Loria-Castellanos5,e
1Directorate of Health Education and Research, High Specialty Medical Unit, Specialty Hospital of Puebla, National Medical Center “Gral. of Div. Manuel Ávila Camacho, Mexican Social Security Institute, Puebla, Mexico.
2College of Sciences and Humanities, Autonomous University of Mexico City, CDMX, Mexico.
3Department of Colon and Rectal Surgery, Siglo XXI National Medical Center, Specialty Hospital “Dr. Bernardo Sepúlveda Gutiérrez”, Mexican Social Security Institute, CDMX, Mexico.
4Department of Inflammatory Bowel Disease, Siglo XXI National Medical Center, Specialty Hospital “Dr. Bernardo Sepúlveda Gutiérrez”, Mexican Social Security Institute, CDMX.
5Special Events Directorate, Mexican Institute of Social Security, CDMX.
aColoproctologist Surgeon
bColoproctologist Surgeon
cSpecialist Physician in General Surgery
dGeneral Physician
eSpecialist Physician in Emergency Medicine
fMaster in Medical Sciences and Research
ABSTRACT
Introduction: Biological treatment is currently used as an alternative for the treatment of ulcerative colitis in patient's refractory to conventional treatment. Objective: To evaluate biological treatment in patients with ulcerative colitis refractory to conventional treatment in a third-level hospital care. Methods: Descriptive, retrospective, longitudinal study in patients with ulcerative colitis refractory to conventional treatment and who received biological treatment. The slices were evaluated at three moments: basal state (without biological therapy), at six and twelve months from the beginning of the biological treatment. Descriptive statistics were used to characterize the population in general; subsequently, the off points were described with their respective variables. Results: Eighteen patients with a mean age of 41.2 years were included. The evaluations, in a basal state, at six and 12 months; showed the presence of blood in stools and abdominal pain in 94.4%, 22.2%, and,% respectively, hemoglobin concentration >10.5 g/dl in 50%, 83.3%, and 88.9%; serum albumin concentration >3.2 g/dl in 72.2%, 83.3%, and 88.9% and visual endoscopic Mayo scale 38.9%, 33.3%, and 16.7% presented in May 2 and 61.1%, 16.7% and 1.7% in May 3. The histological activity in the baseline evaluation reached a severe level (11.1%), while in assessments at six and 12 months, it reached moderate at 55.6% and 27.8%, respectively. Conclusions: Biological therapy as a treatment in patients with ulcerative colitis showed improvement in clinical, biochemical, endoscopic and histological manifestations, so far none with deep remission of the disease, no adverse reactions to treatment have been presented.
Keywords: Colitis, Ulcerative; Treatment; biological; Endoscopies; Clinical. (Source: MeSH NLM).
RESUMEN
Introducción: El tratamiento biológico es una alternativa para manejar la colitis ulcerativa en pacientes refractarios al tratamiento convencional. Objetivo: Evaluar el tratamiento biológico en pacientes con colitis ulcerativa refractarios al tratamiento convencional en un hospital de 3er nivel de atención. Métodos: Estudio descriptivo, retrospectivo, longitudinal en pacientes con colitis ulcerativa refractarios al tratamiento convencional y que recibieron tratamiento biológico. Las cortes se evaluaron en tres momentos: estado basal (sin terapia biológica), a los seis y doce meses de inicio del tratamiento biológico. Se utilizó estadística descriptiva para la caracterización de la población en general, posteriormente los tres puntos de corte se describieron con sus respectivas variables. Resultados: Se incluyeron 18 pacientes con edad media de 41,2 años. Las evaluaciones, en un estado basal, a los seis y 12 meses; demostraron presencia de sangre en las evacuaciones y dolor abdominal en 94,4%, 22,2% y 11,1% respectivamente, concentración de hemoglobina >10,5 g/dl en 50%, 83,3% y 88,9%; concentración sérica de albúmina >3,2 g/dl en 72,2%, 83,3% y 88,9% y escala visual endoscópica de Mayo 38,9%, 33,3% y 16,7% presentaron Mayo 2 y 61,1%, 16,7% y 1,7% Mayo 3. La actividad histológica en la evaluación basal llego hasta un nivel severo (11,1%), mientras que en evaluaciones a seis y 12 meses llegaron hasta moderada en un 55,6% y 27,8% respectivamente. Conclusiones: La terapia biológica en pacientes con colitis ulcerativa refractaria demostró mejoría en manifestaciones clínicas, bioquímicas, endoscópicas e histológicas. No se registró remisión profunda de la enfermedad, ni reacciones adversas al tratamiento.
Palabras Clave: Colitis Ulcerosa; Tratamiento; Biológico; Endoscópica; Clínica.(fuente: DeCS BIREME).
INTRODUCTION
Ulcerative colitis (UC) is chronic, nonspecific inflammation of the intestine characterized by periods of disease activity and remission. Its diagnosis is based on a series of clinical, endoscopic and histological criteria. UC usually predominates in adult patients between 30 and 40 years of age(2). In Mexico, the mean number of new UC cases increased from 28.8 from 1987 to 1996 to 76.1 from 1997 to 2006 (3). The incidence and prevalence have similar behavior in the rest of the world (4). Presentation and development are affected by environmental changes (5).
The main goal of treatment was to induce and maintain long-term remission. According to the Montreal scale, mild, moderate, or severe activity determines the treatment (6-9).
Treatment for mild activity is the use of 5-aminosalicylic acid (5-ASA) (10), adding steroids (for example, prednisone 40-60 mg per day), or equivalent in case of resistance (11). In moderate activity, it is recommended to add thiopurines (azathioprine or mercaptopurine) to the treatment, which can also be used in steroid-dependent patients (12). For severe activity, biological therapy is used (adalimumab, infliximab, among others), and recently the Janus kinase inhibitor (JAK) for rescue. Treatment with intravenous steroids or cyclosporine is usually started for remission induction. This therapy aims to maintain the remission of the disease (13-15). The use of infliximab with azathioprine has a better response in steroid-dependent patients, achieving remission after 16 weeks of treatment (16,17).
Surgical treatment is reserved for emergencies or the presence of premalignant and malignant lesions (18,19).
The use of biological therapy has been proposed in cases of resistance to primary treatment (19) , but the information is still scarce.
So far, there are no predictors of the real behavior of the disease, which help improve treatment and even prevention. Neither is the study of management in case of persistence despite treatment. Hence the importance of evaluating the response to biological therapy, given the lack of long-term studies.
The objective of this study was to evaluate the results of the biological treatment at 12 months in patients with UC refractory to conventional treatment in the Coloproctology Service of a tertiary care hospital in Mexico City, Mexico.
METHODS
Design and study area
A descriptive, retrospective, longitudinal study was carried out in patients with UC who were refractory to conventional treatment and who received biological treatment in the Coloproctology Service of a tertiary care hospital.
Population and sample
The records of patients diagnosed with UC in the Coloproctology Service were reviewed. Identified cases with a confirmed diagnosis of UC, who were under biological therapy (adalimumab or infliximab) for 12 months, previously refractory to conventional treatment (mesalazine, azathioprine, and steroids), of any sex and age, who had endoscopic follow-up were included. , biochemical, clinical, and histopathological.
Variables and instruments
The results of the evaluations were recorded: baseline, six and 12 months after the start of biological therapy, of clinical, biochemical, endoscopic, and histological manifestations.
After the baseline evaluation, biological therapy with tumor necrosis factor inhibitors (anti-TNF) such as infliximab or adalimumab was started. Azathioprine or 5-ASA was used as a compliment.
Information was collected on: age, gender, height, weight, body mass index (BMI), history of diabetes mellitus and arterial hypertension, time of illness, smoking, alcoholism, and appendectomy. Clinical characteristics include bowel movements per day, presence of mucus and blood in stool, abdominal pain, extraintestinal manifestations (arthralgia, myalgia, uveitis, and cirrhosis), and admission to the hospital for associated complications were recorded at each evaluation. The biochemical characteristics recorded were serum hemoglobin, albumin and C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR). Endoscopically, the rating was evaluated according to the Mayo endoscopic scale and the degree of histopathological activity.
The following scales were applied:
● Disease activity was measured, with nine clinical and biochemical characteristics: number of bowel movements per day, blood in stool, fever, tachycardia, hemoglobin, ESR, total leukocytes, serum albumin, and potassium (20,21).
● The Mayo endoscopic scale for UC evaluates its activity through the frequency of bowel movements, bleeding characteristics, endoscopic findings of the mucosa and medical assessment. The activity is divided into 0) normal, mucosa without lesions or in an inactive phase; 1) mild, mild erythema, decreased vascular pattern, mild friability; 2) moderate, marked erythema, absence of vascular pattern, friability, punctate erosions or ulcers; and 3) intense, spontaneous bleeding and ulcerations (7,21,22).
● Histologic activity for UC is classified as no activity, mild activity, moderate activity, and severe activity. It is reported according to the anatomopathological study.
● All patients are questioned about symptoms added to UC. The records were intentionally searched for any clinical condition related or not to the application of the medications.
Procedures
The biological treatment consisted of administering Infliximab or Adalilumab according to the following schemes:
● The administration of Infliximab is at zero, two and six weeks and subsequently every eight weeks at a dose of five to ten mg/kg intravenously.
● Adalimumab was administered at doses of 160/80 mg or 80/40 mg subcutaneously every two weeks (23,24).
● Azathioprine was applied at 2.5-3 mg/kg/day (25).
Statistical analysis
It was performed using descriptive statistics. Measures of central tendency and dispersion were used for the quantitative variables. The statistical program SPSS version 25.0 from IBM was used.
Ethical aspects
The present study complied with the ethical principles for the incorporation of patients in the study, handling personal data with strict confidentiality. All patients signed an informed consent letter when applying the quality of life scale. The protocol was approved by the Health Research Committee No. 3 601 of the Mexican Institute of Social Security, with registration number R-2020-3601-090.
RESULTS
Eighteen patients were included with a mean age of 41.2 (±11.8) years, ten (55.6%) women and eight (44.4%) men. The mean height was 1.61 (±0.8) meters, the mean weight 66.6 (±13.8) kilograms, and the mean BMI was 24.2 (±4) kg/m2. No history of diabetes mellitus or hypertension was found in the population evaluated. A history of smoking was present in two (11.1%) patients, regular alcohol consumption in six (33.3%) patients, and appendectomy in three (16.7%) patients.
Baseline evaluation
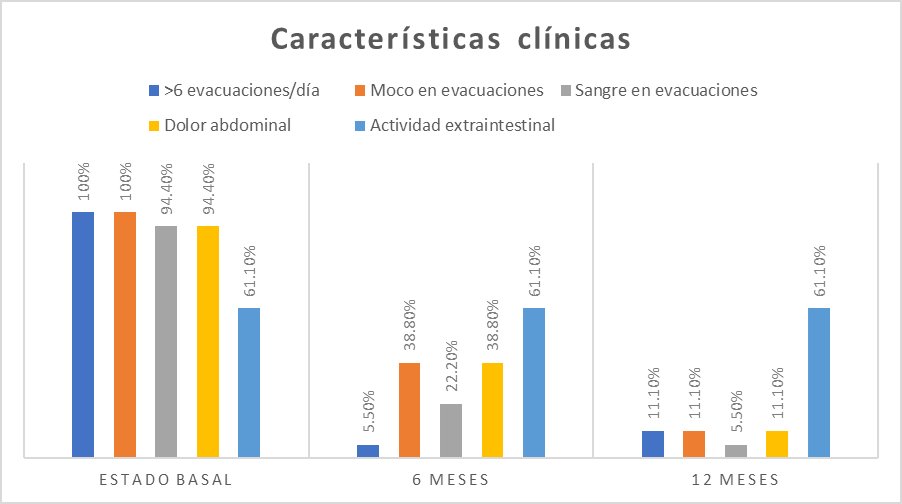
It was observed that the 18 patients presented more than six evacuations per day, 18 reported mucus in evacuations, and 17 registered hematochezia and abdominal pain. In addition, 11 patients presented extraintestinal activity (arthralgia, myalgia, uveitis, and cirrhosis) (Figure 1).
Serum hemoglobin greater than 10.5 g/dl was recorded in 50% of patients, serum albumin greater than 3.2 g/dl in 72.2%, ESR more significant than 10 mm/h in 94.4%, and CRP less than 10 mg/dl in 88.9% (Figure 2).
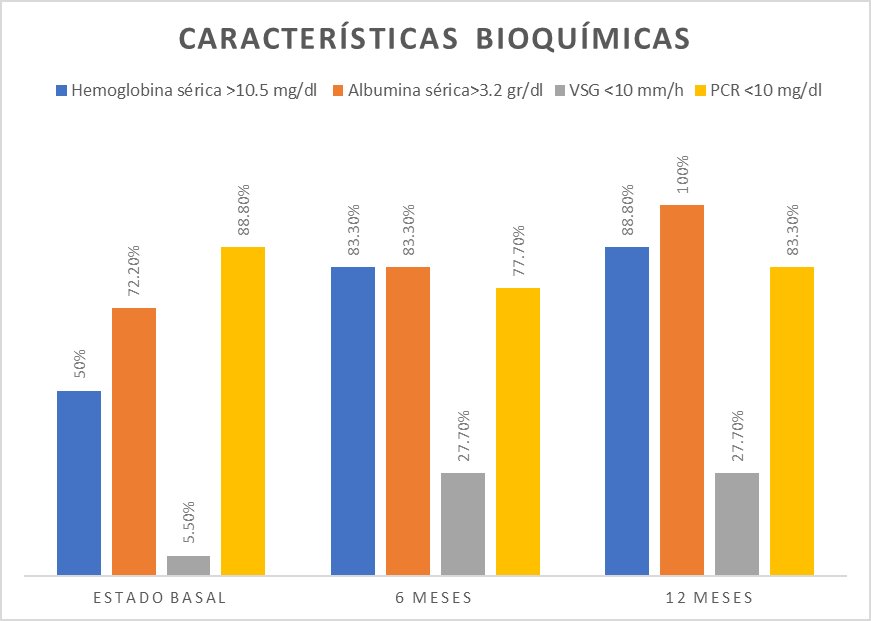
Figure 2: Biochemical characteristics of the patients.
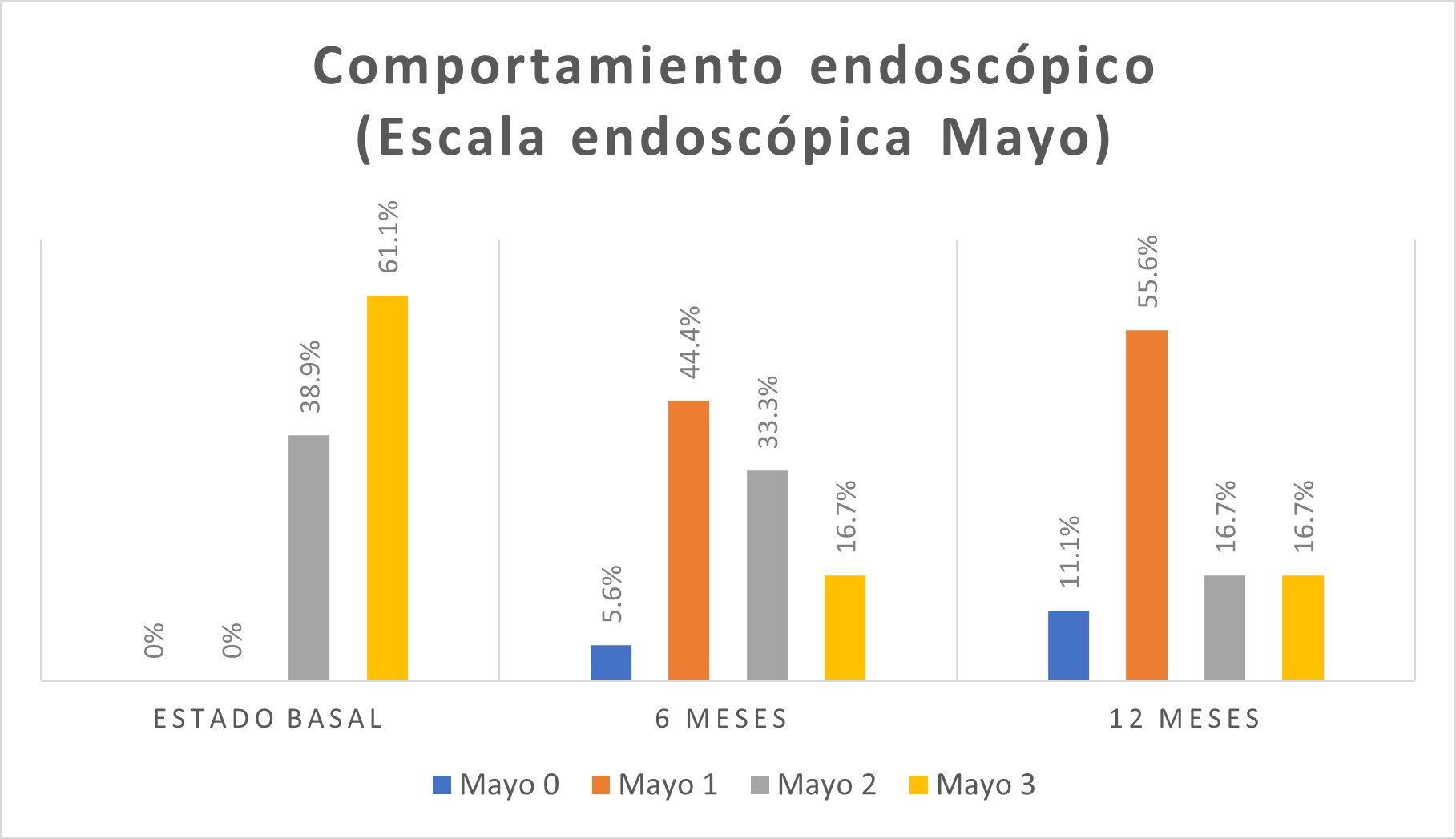
On the Mayo visual endoscopic scale for UC, 11 (61.1%) patients presented a Mayo level of 3 (Figure 3).
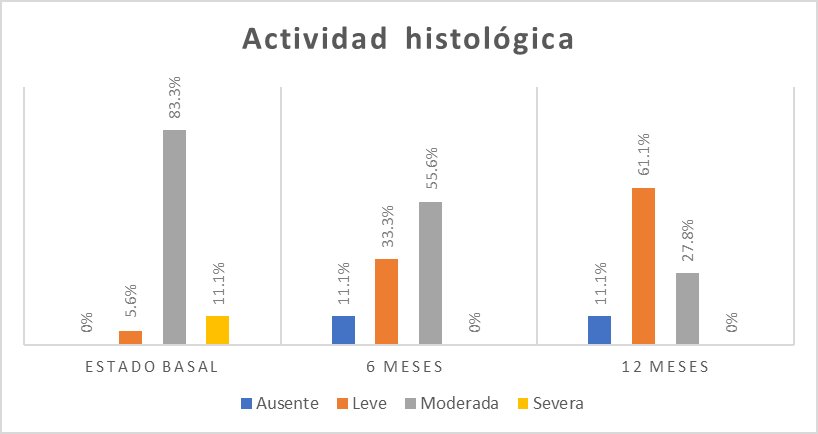
The most frequent degree of histological activity was moderate activity in 15 (83.3%) people (Figure 4).
Regarding hospitalizations, 13 (72.2%) patients did not require hospital management and 5 (27.8%) patients required hospital care before starting the biological treatment. 100% of the patients were refractory to conventional combined treatment to control the disease before starting the biological treatment.
After the baseline evaluation, biological therapy with anti-Tumor Necrosis Factor agents such as Infliximab was started in 10 (55.6%) patients and Adalimumab in 8 (44.4%) patients. Azathioprine was supplemented in 50% of the patients and 5-ASA in 83.3%.
Six-month evaluation
Clinically, 17 patients had fewer than six stools per day; seven had mucus-containing stools, four had blood in the stools, and seven had abdominal pain. Regarding extraintestinal activity, it was present in 11 patients (Figure 1).
A hemoglobin concentration >10.5 g/dl was found in 15 (83.3%) patients; serum albumin concentration was >3.2 g/dl in 15 (83.3%) patients. ESR was <10 mm/h in 5 (27.8%) patients, and CRP <10 mg/dl was found in 14 (77.8%) patients (Figure 2).
In the endoscopic visual scale, a higher frequency of Mayo level 1 was found in 8 (44.4%) patients (Figure 3). The histological activity was moderate in 10 (55.6%) patients (Figure 4).
Evaluation 12 months after starting the biologic
We found 16 patients with less than six bowel movements per day, two patients with mucus in bowel movements, one patient with blood in bowel movements, and two patients with abdominal pain. The extraintestinal activity was present in 11 patients (Figure 1).
Hemoglobin concentration was >10.5 g/dl in 16 (88.9%) patients, and serum albumin concentration >3.2 g/dl was found in 18 (88.9%) patients. ESR was greater than 10 mm/h in 13 (72.2%) patients, and CRP <10 mg/dl in 15 (83.3%) patients (Figure 2).
In the Mayo visual endoscopic scale for UC, in 10 (55.6%) patients, it was Mayo 1 (Figure 3). The mild histological activity was found in 11 (61.1%) patients (Figure 4).
No clinical symptomatology other than the initial UC symptoms that appeared related to the application of the drugs was recorded up to 72 hours after them.
DISCUSSION
Biological therapy has demonstrated efficacy and safety in UC, achieving clinical, endoscopic, histological, and biochemical remission (13,15).
In the follow-up of patients, once the diagnosis is made and treatment started, endoscopic control is recommended at three to six months; clinically, they should be evaluated every three months; once remission is achieved, surveillance can be every six to twelve months (26). The present work was carried out through a basal state and two cuts at six and twelve months, analyzing four aspects: clinical, biochemical, endoscopic and histological.
The histological cure is the complete remission of UC and is associated with a reduction in future complications in clinical practice. In this study, an adequate histological response was obtained, and one patient achieved complete histological remission but not deep remission, which consists in the absence of clinical, endoscopic, histological and biochemical activity.
Infliximab therapy is considered the first line of treatment for UC refractory to conventional treatment and severe activity. It induces and maintains remission up to 52 weeks after its onset (27). Infliximab treatment slightly predominated in this study. Most of the patients registered sustained response, which consists of the absence of relapses during treatment.therapy was used combo, which consists of the use of the biological (Infliximab/Adalimumab), added the use of an azathioprine-type immunomodulator.
Infliximab shows an 80% advantage over placebo in a five-year, with a 50% reduction in the risk of colectomy in patients with severe ulcerative colitis. In the present study, a superior response to biological therapy with infliximab is also observed at six and 12 months in relation to conventional therapy, also adding to the perception of the quality of life of our patients.
Adalimumab induces and maintains remission in 40 to 59.9% of patients with moderate to severe activity in a follow-up of four years (29,30). The response with adalimumab in this study was similar to that observed with infliximab, a therapy combo. Follow-up was recorded at 12 months and is currently continuing.
The immediate adverse reactions associated with the use of infliximab and adalimumab are very similar. They include pruritus, edema, urticaria, hypotension or hypertension, bradycardia or tachycardia, headaches, fever, and anaphylactic shock. Arthralgias, myalgias, urticaria, rashes, fever, or headaches may occur 21 hours after administration. Adverse effects associated with azathioprine are fever, arthralgia, skin rash, malaise, nausea, acute pancreatitis, leukopenia, thrombocytopenia, anemia, infections, and in some cases, hepatitis or tumors (22). No adverse effects were recorded in the present study. This argues in favor of the safety of the drug regimens used. However, its presentation can occur in larger samples.
A comparison of a formal assessment of the quality of life before and after the start of biological therapy is necessary for more robust conclusions. Expanding the sample and longer-term follow-up is also desirable to show the differences between both treatments.
In conclusion, the clinical, endoscopic, biochemical, and histological response in patients who were refractory to conventional treatment was satisfactory. None of them have presented adverse events, nor have patients been hospitalized.
Authorship contributions: The authors participated in the design of the article, analysis and interpretation of data, writing of the article, critical review of the article and approval of the final version.
Funding sources: This project did not obtain funding from any institution or person.
Conflicts of interest: The authors declare no competing conflicts of interest.
Received: May 31, 2022
Approved: April 26, 2022
Correspondence: Arturo García Galicia.
Address: Calle 2 norte # 2004. Colonia Centro. CP 72000. Puebla, Puebla-Mexico.
Telephone number: +521 2222384907
E-mail: arturo.garciaga@imss.gob.mx
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
REFERENCES