ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i3.5027
ANAPLASTIC LARGE T-CELL LYMPHOMA: 10-YEAR EXPERIENCE AT THE NATIONAL INSTITUTE OF NEOPLASTIC DISEASES, LIMA – PERU
LINFOMA DE CÉLULAS GRANDES T ANAPLÁSICO: EXPERIENCIA DE 10 AÑOS EN EL INSTITUTO NACIONAL DE ENFERMEDADES NEOPLÁSICAS, LIMA - PERÚ
Cristian Pacheco1,a, Mónica Calderón2,a, Carlos Barrionuevo3,a, Henry Gomez Moreno2,a
1Department of Oncological Medicine National Institute of Neoplastic Diseases. Lima Peru.
2Biomedical Sciences Research Institute. Ricardo Palma University, Lima Peru.
3Department of Pathological Anatomy of the INEN.
aPhysician Assistant
ABSTRACT
Introduction: Anaplastic Large T-Cell Lymphoma is an infrequent pathology, determined by the expression of CD30, with different characteristics in its presentation and being more aggressive according to ALK expression. Objectives: The present study seeks to determine the epidemiological, clinicopathological, and prognostic characteristics of patients with Anaplastic Large T-Cell Lymphoma. Methods: Descriptive, retrospective study of patients diagnosed with Anaplastic Large T-Cell Lymphoma of the National Institute of Neoplastic Diseases (INEN) between 2006 and 2016. Results: The pathology of 86 patients was analyzed and reviewed, 57% were men and 33 % women; of the total population, 21.9% were positive for ALK, 48 of the patients were found in CD I and II, and 36 between stages III and IV. 57 patients had low or low-intermediate risk, while 26 had high-intermediate and high risk. The estimated overall survival was 40.8% at 5 years; in the group of patients with ALK + it was 67.4%, and in the group with ALK-, it was estimated at 30.2%. Conclusions: Anaplastic Large T-Cell Lymphoma is an aggressive disease with a heterogeneous distribution to age and slightly more frequent in males, with ALK and the international prognostic index as important prognostic factors.
Keywords: Anaplastic Large Cell Lymphoma, Anaplastic Lymphoma Kinase, Survival rate. (fuente: MeSH NLM).
RESUMEN
Introducción: El Linfoma de Células Grandes T Anaplásico es una patología infrecuente, determinada por la expresión del CD30, con diferentes características en su presentación y ser de carácter más agresivos de acuerdo a la expresión del ALK. Objetivos: El presente estudio busca determinar las características epidemiológicas, clinicopatológicas y pronóstico de los pacientes con Linfoma de Células Grandes T Anaplásico. Métodos: Estudio descriptivo, retrospectivo de pacientes diagnosticados con Linfoma de Células Grandes T Anaplásico del Instituto Nacional de Enfermedades Neoplásicas (INEN) entre los años 2006 al 2016. Resultados: Se analizaron y revisaron la patología de 86 pacientes, 57% fueron hombres y 33% mujeres, de la población total 21,9% fueron positivos para ALK. 48 de los pacientes se encontraron en EC I y II y 36 entre estadios III y IV. 57 pacientes presentaban riesgo bajo o intermedio bajo mientras que 26 entre riesgo intermedio alto y alto. La sobrevida global estimada fue 40,8% a los 5 años, en el grupo de pacientes con ALK + fue 67,4% y en el grupo con ALK- se estimó en 30,2%. Conclusiones: El Linfoma de Células Grandes T Anaplásico es una enfermedad agresiva, con distribución heterogénea respecto a la edad y ligeramente más frecuente en varones, con el ALK y el índice pronóstico internacional como factores pronósticos importantes.
Palabras Clave: Linfoma Anaplásico de Células Grandes, Quinasa de Linfoma Anaplásico, Tasa de supervivencia. (fuente: DeCS BIREME).
INTRODUCTION
Anaplastic Large T-Cell Lymphoma (ALCL), initially described in 1985 (1), belongs to the T-cell family and is a unique type of lymphoma strongly expressing the CD30 antigen. This neoplasm is relatively infrequent, found in around 2 to 8% of all non-Hodgkin's lymphomas, between 10% to 20% of high-grade lymphomas, and 16% of T-lymphomas (2).
According to the World Health Organization (WHO) (3), the expression of Anaplastic Lymphoma Kinase 1 (ALK1) determines the presence of two different entities: ALCL ALK positive (+), which represents 70%, it is more common in children and adolescents, males with a less aggressive course and better prognosis; and ALCL ALK negative (–), considered a provisional entity, more common in adults, without gender predominance, low incidence of systemic symptoms and worse prognosis (4,5). Both entities share similar characteristics, especially the morphological ones and the overexpression of CD30, which allows them to be differentiated from the rest of the peripheral T-cell lymphomas.
The International Prognostic Index (IPI) is a tool used as a predictive index for the survival of patients with non-Hodgkin's lymphoma. In recent years, a prognostic index for T-cell lymphoma (PIT) has been introduced, which includes the compromise of bone marrow, which is rare in ALCL, both indices are similar in this pathology (6), so it has not yet been fully approved for ALCL.
Treatment continues to be combined chemotherapy associated with anthracyclines (7), which reaches up to 80% complete response, with relapse rates of approximately 25%; in patients with residual disease (especially in mediastinal lymphomas) radiotherapy is indicated.
The present study seeks to determine the epidemiological, clinical-pathological, and prognostic characteristics of patients older than 14 years with ALCL, diagnosed at the National Institute of Neoplastic Diseases (INEN) in Lima, Peru 2006 and 2016. This will allow to know the form of presentation in our population and thus be able to take the best management and monitoring option. In addition, it will serve as the basis for future analyzes and improvements in treatment.
METHODS
Design and study area
Descriptive, retrospective study of a patient diagnosed with Anaplastic Lymphoma, diagnosed at the INEN Lima, Peru, between 2006 and 2016.
Population and sample
91 medical records were analyzed, having as inclusion criteria all the patients 14 years of age or older, with a pathological diagnosis of Anaplastic Large Cell Lymphoma, of any location and without previous treatment. Two patients under 14 years of age were excluded, two referred as anaplastic lymphoma but with no pathology in the INEN, and one with previous treatment in another institution. Therefore, we worked with the 86 medical records that met the inclusion criteria.
Variables and instruments
The anatomopathological diagnosis of Anaplastic Large Cell Lymphoma was made under the recommendations of the WHO (3), which describes the presence of large, pleomorphic lymphoid cells with abundant cytoplasm and frequent presence of a horseshoe nucleus (hallmark cell), with expression intense CD30, and immunopositivity or not for ALK, this diagnosis excludes B lymphomas with CD30 expression (8,9).
In addition, the epidemiological characteristics, staging and survival of the patients were analyzed.
Procedures
The pathological anatomy results of each medical record, which included immunohistochemical studies with expression mainly of CD30 (Clona Ver-Hz, Dako, Denmark), ALK (Clona ALK1, Dako, D), CD3 (Policlonal Dako Denmark), EMA (Clona E29, Dako D), CD45 (Clona 2B11, Dako, Denmark) and CD20 (Clona L26, Dako, D), were re-evaluated and confirmed by an expert pathologist.
The epidemiological characteristics evaluated were age, sex, origin, and patient quality of life using the Eastern Cooperative Oncology Group (ECOG) scale, date of diagnosis, date of the last consultation, and survival status.
For staging, imaging studies were analyzed, including X-rays and tomography, bone marrow aspiration with bone biopsy, all re-evaluated by radiologists and pathologists from our Institution, and then used the Ann Arbor Staging System (10).
The following parameters were used to determine the International Prognostic Index (IPI) (11), older than 60 years of age, patient quality of life, number of lymph node sites, clinical stage, extranodal involvement, and lactate dehydrogenase above the normal value.
The patients received CHOP or CHOP-like chemotherapy as a treatment scheme (12). Response to treatment was evaluated using the Response criteria of the American Society of Clinical Oncology (13).
Statistical analysis
A descriptive analysis was performed through frequencies, percentages, and summary measures (mean and range). For the analysis of progression-free survival, the follow-up time was considered from the start date of the first treatment to the date of recurrence/progression, death or last control; For the analysis of overall survival, the follow-up time was considered from the start date of the first treatment to the date of death or last control. In estimating survival, the Kaplan-Meier method was used, and differences between survival curves were tested with the logrank test. The selection of factors that increase the risk of death was carried out through the Cox regression model. A value p<0.05 was considered a significant difference and risk. Statistical analysis was performed using the SPSS 22.0 statistical system.
Ethical aspects
Confidentiality of the identity of the patients and the information in the medical records was guaranteed; likewise, the guidelines of the Declaration of Helsinki were met.
RESULTS
The characteristics of the patients are detailed in Table 1.
Table 1. Characteristics of the patients.
| Characteristics | n (%) |
|---|---|
| Age, years | |
| Mean / Range | 43 / [15-88] |
| Sex | |
| Male | 49 (57.0) |
| Female | 37 (43.0) |
| Quality of Life (ECOG) | |
| 1 | 50 (58.1) |
| 2 | 26 (30 .2) |
| 3 | 6 (7.0) |
| 4 | 4 (4.7) |
| LHD (IU / L) | |
| Normal | 33 (38.4) |
| High | 48 (55.8) |
| SEL | 5 (5.8) |
| Bulky (tumor ≥10cm) | |
| Yes | 25 (29.1) |
| No | 57 (66.3) |
| SEL | 4 (4.7) |
| ALK | |
| Negative | 53 (61.6) |
| Positive | 18 (20.9) |
| SEL | 15 (17.4) |
| Clinical Stage | |
| I | 19 (22, 1) |
| II | 29 (33.7) |
| III | 12 (14.0) |
| IV | 24 (27.9) |
| SES | 2 (2.3) |
| IPI | |
| Low | 34 (39.5) |
| Low Intermediate | 23 (26.7) |
| High Intermediate | 20 ( 23.3) |
| High | 6 (7.0) |
| SES | 3 (3.5) |
| Chemotherapy | 67 (79.6) |
| Radiotherapy | 3 (3.4) |
| No treatment | 16 (17.0) |
| Infectious | 5 |
| Family disagreement | 4 |
| Lost sight/OI | 6 |
| Total | 86 (100) |
DHL: Lactic dehydrogenase.
There were 86 patients in the study with a mean age of 43 years (range, 15 to 88 years), of whom 49 (57.0%) were men and 37 (43.0%) were men. .0%) women. More than 50% of patients had a good ECOG 1 performance status. According to DHL levels, 48 (55.8%) patients had elevated status. According to ALK expression, 18 (20.9%) patients were positive, 53 (61.6%) were negative, and 15 (17.4%) did not have this result. According to the stage, 19 (22.1%) were stage I, 29 (33.7%) were stage II, 12 (14.0%) were stage III, and 24 (27.9%) were stage IV, in 2 ( 2.3%) patients, the stage is not specified. According to the international prognostic index, 34 (39.5%) were low risk (BR), 23 (26.7%) low intermediate (IB), 20 (23.3%) high intermediate (IA), and 6 (7 .0%) high risk (AR), in 3 (3.5%) patients the index is not specified because they do not have the complete data recorded in the history. 67 patients (79.6%) received chemotherapy, 3 (3.4%) radiotherapy, and 16 (17%) did not receive treatment; the causes were sepsis (5 patients), no desire for therapy (4 patients), and transfers to another institution ( 6 patients). Of the patients who received chemotherapy, 24 (35.8%) achieved complete response (CR), 10 (14.9%) partial response (PR) and 33 (49.3%) no response and/or disease progression.
Regarding the distribution of ALK+ and ALK- cases by age groups, the T-test for independent samples shows that the average age for ALK + patients was significantly lower than that of ALK - patients (27.9 years vs. 47.4 years p<0.05). In the group of ALK+ patients, 25% were under 17 years of age, 50% were under 22.5 years of age, and 25% over 34 years of age. 01 distant value to the rest (atypical case) of a 58-year-old patient was identified. In the ALK- group, 25% were under 30 years old, 50% were under 50 years old, and 25% were over 62 years old; no atypical cases were found.
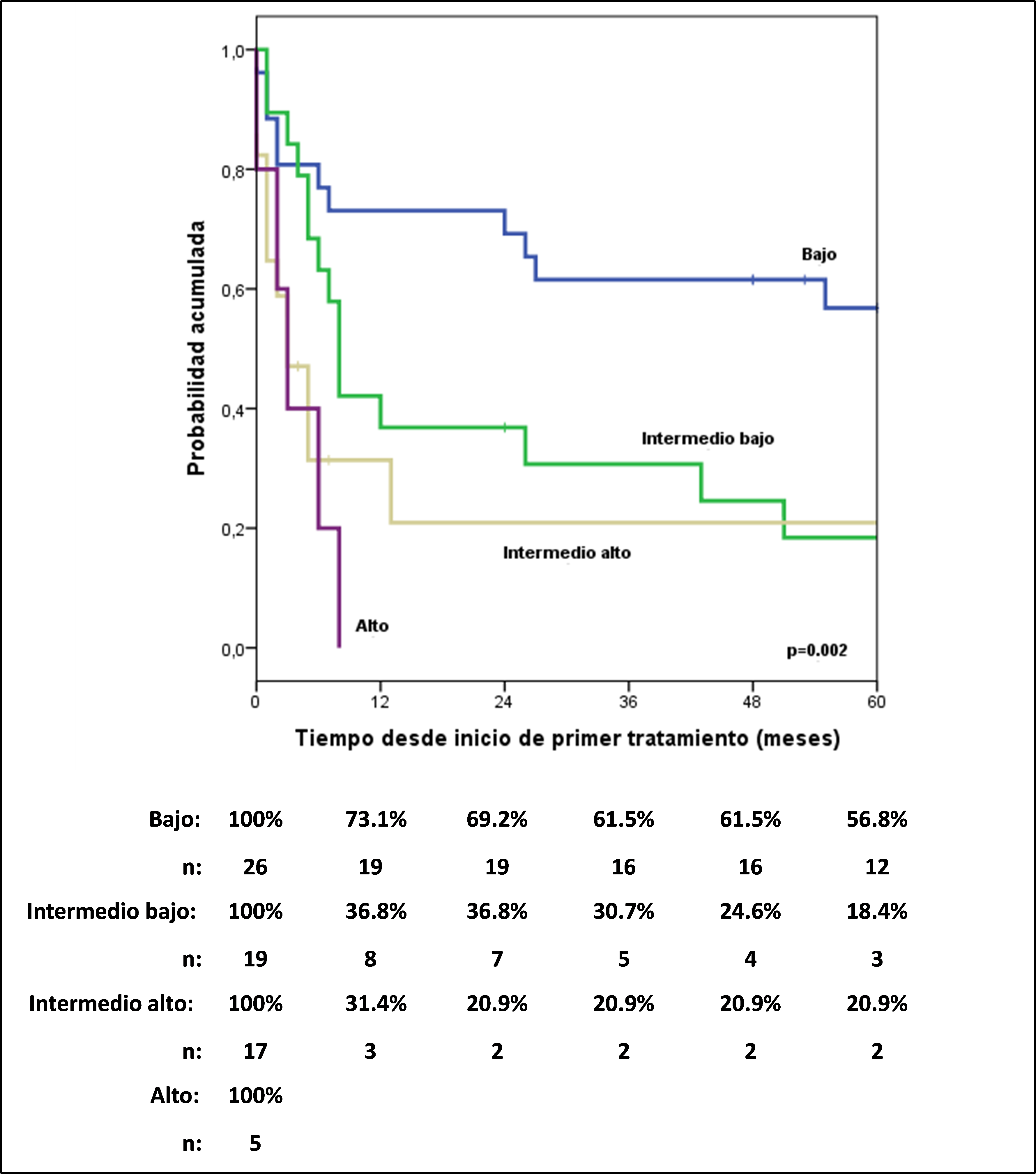
The 5-year progression-free survival (PFS) ranges for ALCL ALK+ and ALK- patients were 62.2% and 20.7%, respectively (HR: 4.52; 95% CI, 1.6-12.74). ; p=0.004). The International Prognostic Index (IPI) (Figure 1) identified different risk groups across the entire cohort.
5-year PFS was 56.8% for low IPI, 18.4% for low-intermediate, 20.9 % for intermediate IPI, and 0% for high risk; something similar occurred with the PIT, which also showed a high risk of mortality in the high and intermediate-high group compared to the low and intermediate-low group (HR: 6.25; 95% CI, 2.96-12.79; p= 0.05).
When performing multivariate analysis, including ALK characteristics, DHL, stage and IPI, and choosing the most influential characteristics, it turned out that an ALK negative has approximately 6 times more risk of progression compared to an ALK positive, and that the low intermediate IPI, high and high intermediate have 3, 6 and 6 times more risk of progression compared to a low IPI, respectively (Table 2).
Table 2. Univariate and multivariate analysis of the association of characteristics with progression-free survival.
| Univariate | Multivariate | Multivariate* | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | n | p | HR | p | HR | p | HR |
| ALK | |||||||
| Positive | 15 | 1,00 | 1,00 | 1,00 | |||
| Negative | 43 | 0,004 | 4,52 (1,6-12,74) | 0,001 | 7,28 (2,37-22,33) | 0,001 | 5,97 (2,02-17,59) |
| DHL | |||||||
| Normal | 27 | 1,00 | 1,00 | ||||
| High | 39 | 0,009 | 2,32 (1,23-4,36) | 0,404 | 1,48 (0,59-3,69) | ||
| Stage | |||||||
| I | 14 | 1,00 | 1,00 | ||||
| II | 25 | 0,437 | 1,42 (0,59-3,44) | 0,914 | 1,06 (0,36-3,17) | ||
| III | 8 | 0,338 | 1,71 (0,57-5,1) | 0,671 | 0,75 (0,19-2,84) | ||
| IV | 21 | 0,028 | 2,74 (1,12-6,73) | 0,299 | 1,92 (0,56-6,55) | ||
| IPI | |||||||
| Low | 26 | 1,00 | 1,00 | 1,00 | |||
| Low Intermediate | 19 | 0,034 | 2,28 (1,07-4,88) | 0,375 | 1,69 (0,53-5,38) | 0,022 | 2,67 (1,15-6,22) |
| Intermediate High | 17 | 0,002 | 3,47 (1,58-7,65) | 0,084 | 3,36 (0,85-13,24) | <0,05 | 6,14 (2,34-16,12) |
| High | 5 | 0,002 | 5,64 (1,92-16,61) | 0,107 | 3,45 (0,77-15,52) | 0,003 | 5,58 (1,76-17,68) |
Regarding overall survival, the median follow-up time was 72 months. Estimated overall survival at 1, 3, and 5 years was 61.4%, 46.8%, and 40.8%, respectively. In the group of patients with ALK+, overall survival at 1, 3, and 5 years was estimated at 85.6%, 77%, and 67.4%, respectively; and in the group with ALK- it was estimated at 54.1%, 36.3% and 30.2% at 1, 3 and 5 years, respectively. A significant difference was found between both groups. In the group of patients with normal LDH, overall survival at 1, 3, and 5 years was estimated at 89.1%, 70%, and 61%, respectively. In the group with high LDH it was estimated at 41.5%, 28.9% and 25.3% at 1, 3, and 5 years, respectively, a significant difference was found between both groups. In the group of patients with stage I, overall survival at 1, 3, and 5 years was estimated at 73.3%, 66.7%, and 66.7%, respectively; in the group, with stage II it was estimated at 71.2%, 53.3% and 42.6% at 1, 3 and 5 years, respectively; in the group of patients with stage III, overall survival at 1, 3, and 5 years was estimated at 75%, 45%, and 22.5%, respectively. In the group with stage IV it was estimated at 36.7%, 24.4% and 24.4% at 1, 3 and 5 years, respectively. A significant difference was found between the groups. In the group of patients with IPI BR, overall survival at 1, 3, and 5 years was estimated at 85.2%, 70.4%, and 65.7%, respectively; in the group with IPI IB it was estimated at 66.9%, 46.3% and 30.9% at 1, 3 and 5 years, respectively; in the group of patients with IPI IA, overall survival at 1, 3, and 5 years was estimated at 31.4%, 15.7%, and 15.7%, respectively; and in the IPI AR group, it was estimated at 0.0% at 9 months. A significant difference was found between the groups (Figure 2).
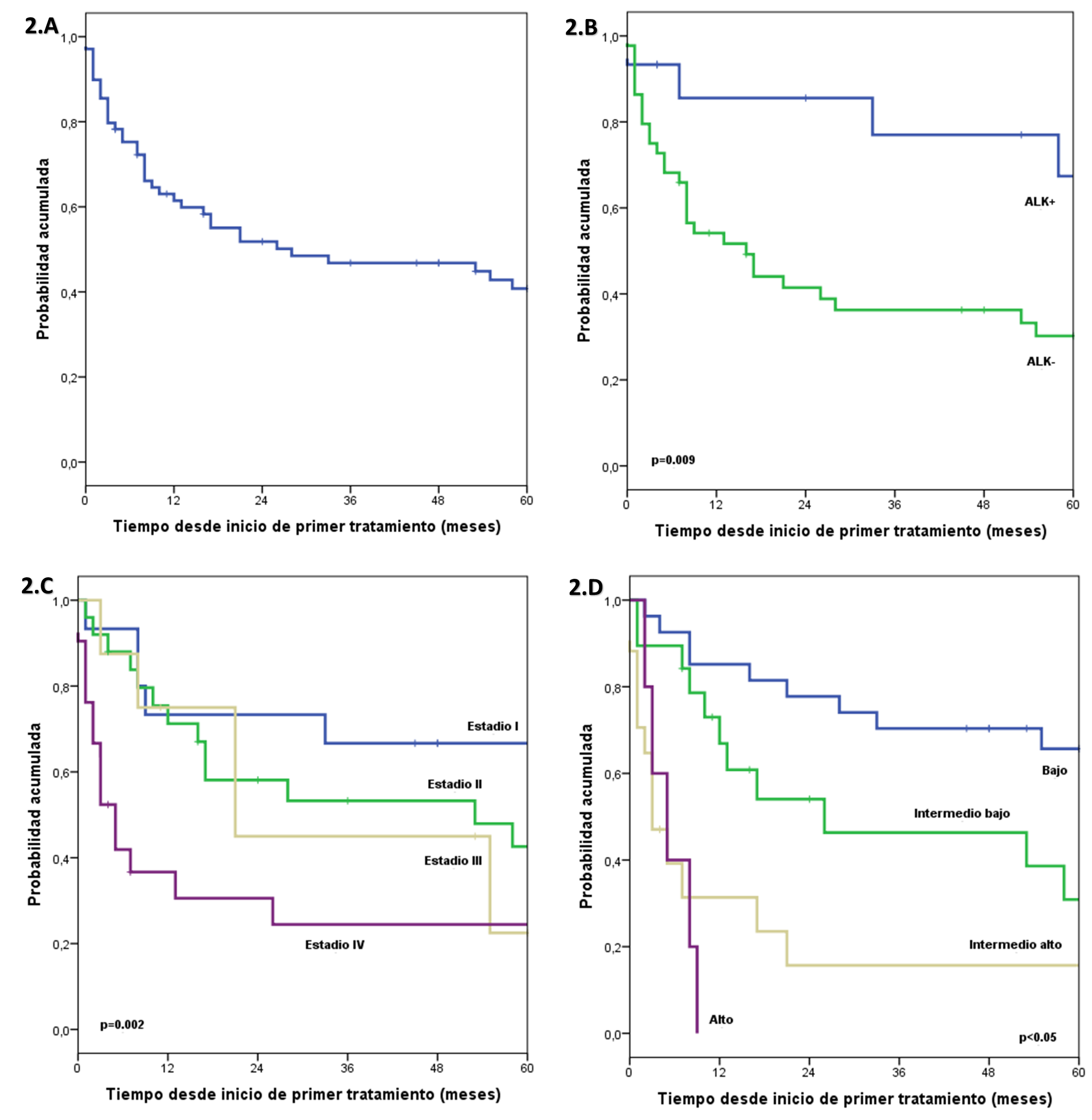
In the multivariate analysis, including the ALK characteristics, DHL, stage and IPI, it was found that ALK-negative has a 5-fold higher risk of death compared to ALK-positive and that the low intermediate, high intermediate and high IPI have 3, 10, and 9 times higher risk of death compared to a low IPI (Table 3).
Tabla 3.Análisis univariado y multivariado de asociación de características con la sobrevida global.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Characteristics | n | p | HR | p | HR |
| ALK | |||||
| Positive | 15 | 1,00 | 1,00 | ||
| Negative | 44 | 0,015 | 3,69 (1,29-10,63) | 0,003 | 5,63 (1,77-17,94) |
| DHL | |||||
| Normal | 28 | 1,00 | |||
| High | 39 | 0,002 | 3,12 (1,53-6,35) | ||
| Stage | |||||
| I | 15 | 1,00 | |||
| II | 25 | 0,206 | 1,94 (0,69-5,38) | ||
| III | 8 | 0,204 | 2,24 (0,65-7,76) | ||
| IV | 21 | 0,005 | 4,31 (1,55-11,96) | ||
| IPI | |||||
| Low | 27 | 1,00 | 1,00 | ||
| Low Intermediate | 19 | 0,07 | 2,22 (0,94-5,28) | 0,032 | 2,98 (1,09-8,08) |
| Upper intermediate | 17 | <0,05 | 5,49 (2,35-12,81) | <0,05 | 10,37 (3,71-28,95) |
| High | 5 | <0,05 | 8,87 (2,82-27,93) | <0,05 | 9,17 (2,64-31,94) |
DISCUSSION
ALCL is a lymphoma with an aggressive and heterogeneous clinical course, with a higher prevalence in children and young adults. Most studies report a better response to treatment in young patients, reaching a response range between 60%-90%. Sibón et al. reported in patients under 40 years of age, survival regardless of ALK status (14,15). Recent analyzes promote high-dose chemotherapy followed by Stem Cell Transplantation to prolong survival time; e there, the importance of the search and identification of prognostic factors for selecting the most aggressive population to offer them more intense and optimal therapy regimens.
In our study, we analyzed the epidemiological, clinicopathological, and prognostic characteristics of patients with ALCL. The median age of 40 years was found, regardless of ALK status, which is somewhat higher compared to other series where the median age was less than 35 years (16,17), the range of men/women was 1, 3:1, similar to other published series with a range of 1.6-1.8:1. Patients with ALK expression were more frequent in the first and second decades of life, with a median age of 22 years, which is lower compared to other publications such as that of Ferrari et al. 2012 (18) where they present median age of 34 years. Other studies, such as the one by Savage et al. (2), found that ALCL ALK positives compromise skin, subcutaneous cellular tissue, and bone marrow. We found 3 patients with visceral, hepatic, and splenic compromise and only 2 patients with the compromise of bone marrow.
Various studies consider ALCL a chemosensitive disease, in some cases even comparable to Diffuse Large B-Cell Lymphoma, to treatment with the indication of starting first-line chemotherapy based on anthracyclines (12) the standard scheme is CHOP, and in cases of relapses with schemes based on platinum. Studies show an overall response between 70% and 80% (19), higher than that found in our study, where it reached 50.7% after the first line of treatment.
In our analysis, it was shown that the complete response achieved after the first line of chemotherapy has a better prognosis and a longer overall survival time compared to patients who only achieved a partial response. The literature indicates that the partial or complete response after the administration of chemotherapy, based on the CHOP or CHOP like scheme, can reach up to 65%; in our study said the response to the first line of treatment reached up to 50.7%. .
The 5-year survival for ALK+ patients was 62.5%, similar to the 60% reported by Savage et al; for ALK - patients it was 29.6% lower than the 36% reported in the same study, evidencing, also described in other studies, that ALCL ALK + is an important favorable factor in survival (2). It has been shown that ALK plays an important lymphomatogenesis(5), strongly suggesting that its inhibition would be sufficient to attenuate the growth and survival of ALCL ALK+ cells, considering it as a potential molecular target in the therapy of this disease and in others where its presence has been demonstrated, such as lung cancer and inflammatory myofibroblastic tumor (20,21). A study showed that the better evolution of patients with ALCL ALK+ may also be associated with higher levels of apoptosis due to chemotherapeutic agents than ALK- cells (22), which would increase the greater responses and better prognosis in these patients.
Other factors that could be considered prognostic factors were also identified: lactic dehydrogenase above the normal value, the International Prognostic Index, and the clinical stage (6), all with statistical significance, while other works do not consider that these are independent factors (23). We found positivity for ALK as a prognostic factor and an international prognostic index with a significant statistical difference.
The limitations of the study have been that it is a retrospective study and that 17% of the population did not receive cancer treatment.
CONCLUSIONS
ALCL is an aggressive disease with a heterogeneous distribution for age and slightly more frequent in males, where ALK expression can be considered a prognostic factor (where its expression determines the existence of two different entities due to the age of presentation and aggressiveness) and the International Prognostic Index. Treatment based on anthracyclines allows obtaining an overall response greater than 50% with overall survival of 38.5% at 5 years.
Authorship contributions:CPR participated in the conceptualization, research, methodology, resources, and writing of the original draft.
MCA participated in the conceptualization, research, methodology, resources, and writing of the original draft.
CBC participated in the conceptualization, research, methodology, resources, and writing of the original draft.
HGM participated in the conceptualization, research, methodology, resources, and writing of the original draft.
Funding sources: Self.
Conflicts of interest: The authors declare no conflict of interest.
Received: June 17, 2022
Approved: July 07, 2022
Correspondence: Mónica Jackelin Calderón Anticona.
Address: Fray Angelicco 506 Apartment 403. San Borja. Lima Peru.
Telephone number: +51 999545990
E-mail: mjca46@hotmail.com
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
REFERENCES