ARTÍCULO DE REVISIÓN
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i4.5053
EFFECTIVENESS OF INTERMITTENT FASTING ON BIOCHEMICAL AND ANTHROPOMETRIC MARKERS IN OBESE ADULTS WITH CARDIOVASCULAR RISK. A SYSTEMATIC REVIEW
EFECTIVIDAD DEL AYUNO INTERMITENTE SOBRE MARCADORES BIOQUÍMICOS Y ANTROPOMÉTRICOS EN ADULTOS OBESOS CON RIESGO CARDIOVASCULAR. UNA REVISIÓN SISTEMÁTICA
Francisca Osses-Carrasco1,a, Francisca Gómez-Zúñiga1,a, Miguel Ángel
López-Espinoza1,b
1Nutrition and Dietetics Program, Faculty of Health, University of Santo Tomas. Talca, Chile.
aBachelor's Degree in Nutrition and Dietetics
bNutritionist, Master in Public Health, Doctorate in Health Sciences
ABSTRACT
Introduction: Obesity is a problem in almost all societies, leading to the search for different methods to combat it. One of them is intermittent fasting (IF), characterized by periods without food intake (16 to 24 hours), limited or no caloric intake, combined with normal eating windows. Objective: The study aims to determine the effectiveness of intermittent fasting on biochemical and anthropometric markers in obese adults. Methods: A systematic review was proposed that aimed to study blinded or open clinical trials of IF interventions, compared to the control group. The response variables were: systolic and diastolic blood pressure, total cholesterol, LDL, HDL, triglycerides, blood glucose, fat mass, weight, waist circumference, BMI, and heart rate. The search and identification of studies was masked. Risks of bias for the Cochrane Collaboration were assessed. They were subjected to meta-analysis (random effect), with R 4.0.0. Results: Six studies were included, of 10 to 48 weeks of intervention with alternate-day fasting and time-restricted feeding, reporting some statistically significant changes for different variables (specify). Conclusion: Intermittent fasting could intervene in the reduction of cardiovascular risk by improving BMI and biochemical parameters.
Palabras Clave: Intermitent fasting, obesity, systematic review, lipid metabolism, body composition, blood pressure (Source: DECS-BIREME).
RESUMEN
Introducción: La obesidad es un problema presente en casi todas las sociedades, lo que ha conllevado a buscar distintos métodos para combatirla. Uno de ellos es el ayuno intermitente (AI), caracterizado por periodos sin ingesta (16 a 24 hr), ingesta calórica limitada o nula, combinada con ventanas de alimentación normal. Objetivo: Determinar la efectividad del ayuno intermitente sobre los marcadores bioquímicos y antropométricos en adultos obesos. Métodos: Se planteó una revisión sistemática que postuló estudiar ensayos clínicos enmascarados o abiertos de intervenciones de AI, comparado con grupo control. Las variables de respuesta fueron: presión arterial sistólica y diastólica, colesterol total, LDL, HDL y triglicéridos, glicemia, masa grasa, peso, circunferencia de cintura, IMC y frecuencia cardiaca. La búsqueda e identificación de los estudios fue enmascarada. Se evaluaron los riesgos de sesgo de la colaboración Cochrane. Se sometieron a meta-análisis (efecto aleatorio), con R 4.0.0. Resultados: Se incluyeron 6 estudios, de 10-48 semanas de intervención con ayuno en días alternos y alimentación con restricción de tiempo, reportándose algunos cambios estadísticamente significativos para distintas variables (especificar). Conclusión: El ayuno intermitente podría intervenir en la disminución del riesgo cardiovascular por mejoría en IMC y parámetros bioquímicos.
Palabras Clave: Ayuno intermitente; Obesidad; Revisión sistemática; Metabolismo lipídico; Composición corporal; Presión arterial (Fuente: DECS-BIREME).
INTRODUCTION
Worldwide, during the last two decades, obesity has been considered a global public health problem (1,2). It is characterized by an increase in body fat, with a BMI equal to or greater than 30 kg/m2 (3), associated with multiple well-described factors such as Socioeconomic level (4), Years of education (5), Obesogenic environments (6), Food quality (1), Culture (1), Race (7), Sex (3), Genetics (8), Sleep disturbance (9), Drug use (10,11), Stress (12), Hormonal changes (4), Eating in front of screens (13) , and Sedentary lifestyle (14).
Obesity is a frequent problem in countries with different realities. According to the Organization for Economic Cooperation and Development (OECD) in 2017 (15,16) the following countries stand out: United States (40%), Chile (34.4%), Mexico (33.3%), New Zealand (32 .2%), Hungary (30%), Turkey (28.8%), Portugal (28.7%), Canada (28.1%) and Australia (27.9%).
Body weight is controlled by multiple organs, hormones, and metabolic pathways, as well as environmental factors. The Energy Balance Model (EBM) and high-calorie-dense processed foods explain the increase in fat (17). A series of comorbidities such as arterial hypertension, insulin resistance, and diabetes, hypercholesterolemia, among others, are observed (18).
The basis of obesity management is the control of energy balance by reducing intake and increasing energy expenditure (19). To this, it has been added other types of approaches such as intermittent fasting diets, eating patterns where the individual spends a period of time with little or no energy (20). There are variants such as complete fasting on alternate days: food intake on 1 day and then fasting the next day (21); alternate day fasting: consumption of 20-25% of total energy expenditure on fasting days (22); time-restricted eating: meals with a certain energy value during the “food window” (20) ; and finally, religious fasting (23). According to Johnstone (24), intermittent fasting is considered to be less restrictive compared to traditional methods of calorie restriction. The most popular and used variation is 16:8, that is, a 16-hour fast and an 8-hour nutritional window (23). This type of strategy allows positive changes in patients with obesity and comorbidities, maintaining average body weight, and having positive effects on glycemic control due to the decrease in insulin levels (24), according to studies with animals and humans (20).
Even though it may be a potentially interesting future dietary strategy for longevity (23), its study is incipient and the long-term effect of this nutritional strategy is not clear.
Therefore, our study aims to determine the effectiveness of intermittent fasting on biochemical and anthropometric markers in obese adults.
METHODS
Systematic review design
A systematic review of clinical trials based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)(25).
Study Eligibility Criteria
Randomized clinical trials were collected (simple or by balanced blocks), that have hidden the
randomization sequence, with or without blinding. The patients of both sexes who were studied are in an
age range between 18 to 65 years, with obesity (BMI ≥ 30 kg/m2), and patients with cardiovascular
comorbidities (for example metabolic syndrome) were also accepted. The interventions that were studied
are: Alternate-day Fasting (ADF): consumption of 20 to 25% of total energy expenditure on scheduled
fasting days, and normal feeding for 24 hr; time-restricted eating: fasting for a certain time, with a
feeding window. The control group should be characterized by the consumption of a usual diet. The
Intervention time was at least 10 weeks.
The following response variables were considered: systolic and
diastolic blood pressure (mmHg), total cholesterol, LDL, HDL, triglycerides, blood glucose (mg/dl), BMI
(kg/m2), percentage body fat mass, weight (kg), waist circumference (cm), and heart rate (bpm). In the
case of the unit of measure of the biochemical indicators previously indicated described with mmol/L,
they were transformed with conversion factors (26).
Search strategy
Search strategies were developed between June and July 2021. The keywords that were used were extracted from Medical Subject Heading (Mesh) and non-controlled words obtained from the specific language of the topic were added to them.
The following databases were used: Pubmed, Cochrane Library, and Proquest. In addition, the site Clinicaltrials.org was reviewed to find out the status of any unpublished clinical trial. No publication date or language restrictions were applied.
Selection of studies
Two previously masked and trained reviewers identified whether the titles of the clinical trials contained information associated with the research question and eligibility criteria. Each of the reviewers classified the clinical trials as: “included”, “excluded” and “uncertain”. Once the results were opened, potential inconsistencies were evaluated and resolved by consensus with a third reviewer. Then, the available articles were subjected to full reading by the same two previously masked reviewers, to classify studies as “included” or “excluded”. Disagreements were resolved by consensus with a third reviewer.
Data extraction
The same two previously identified reviewers, in a masked manner, extracted from the included articles: author and year of publication; population; intervention characteristics; control group; main results. The CONSORT guideline (27) was applied to facilitate critical reading and extraction of the required information.
Measurement quality
Each researcher individually evaluated the five risks of bias according to the domains suggested by the Cochrane Collaboration (28). Each reviewer rated one of the following 3 alternatives for each risk of bias: “low”, “high” or “unclear”. Once the results were opened, the degree of concordance of the responses was evaluated and in case of disagreement, a third researcher collaborated to reach a consensus response. For this work, the Rev. Manager 5.3 program (29) was applied.
Statistic analysis
Clinical trials with homogeneity underwent meta-analysis to obtain weighted mean differences, with their respective 95% confidence intervals. The random-effects method was applied (intra- and inter-study variability are assumed). The degree of heterogeneity was measured with I2 and Cochran's Q test, with p < 0.10. The Metafor package (30) was applied in R, version 4.0.0. (31).
RESULTS
Search for results
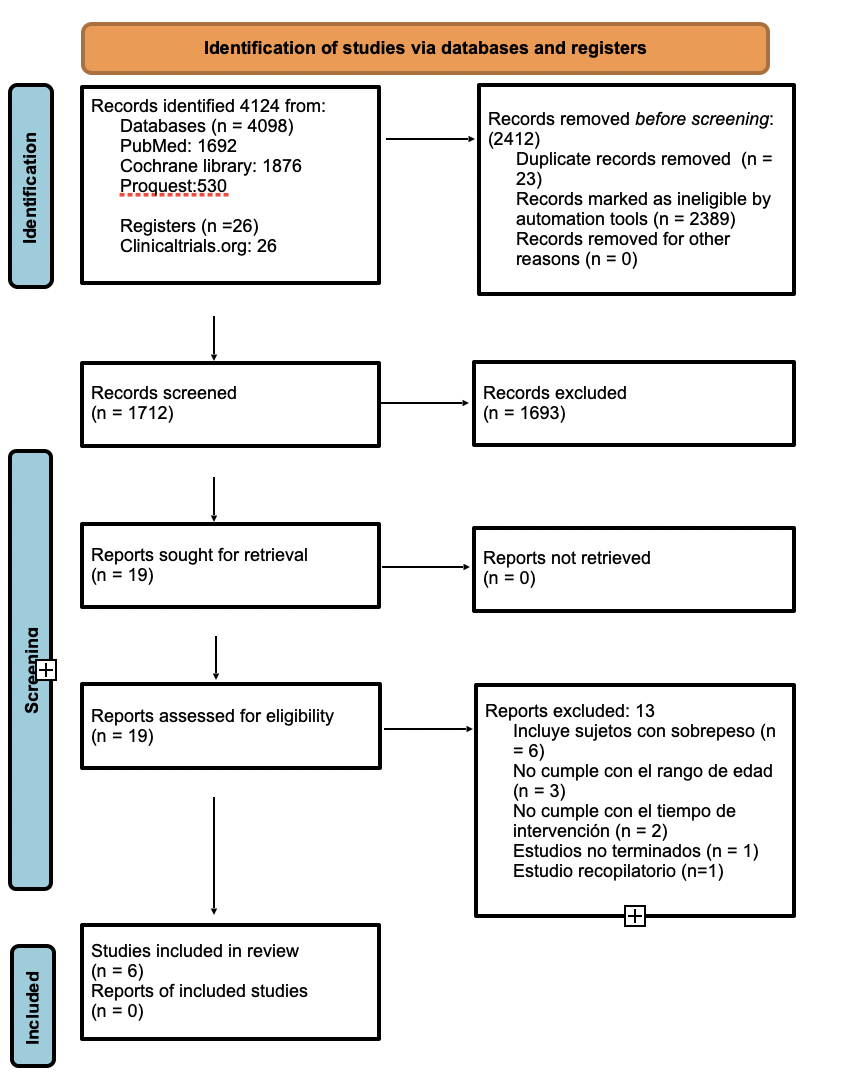
The search process for the articles included is presented in Figure 1.
Six studies published on or before 2021 were included and used for description in the qualitative phase. A meta-analysis of two studies included in this systematic review was performed (34,36).
Characteristics of included studies
The main characteristics of the included studies are available in Table 1. Five studies were conducted in the US (33,34,36-38) and one study in Brazil (35). The number of participants ranged from 16 to 83 participants, the mean ages ranged from 25 to 65 years, and the proportion of female participants ranged from 75% to 88% (33,35), except for one study that included only women (36).
The interventions lasted between 10 and 48 weeks. Five of the studies included alternate day fasting (ADF) interventions, in which subjects consumed 25% of their initial energy needs on the fasting day (24 hr) and ate ad libitum on each alternate feeding day within 24 hr. One study included a time-restricted fasting (TRF) intervention, where subjects consumed food within a 12-hour period, then fasted for the other 12 hours. All the studies presented two groups, except for one study that had 3 interventions and 1 control (37). All studies evaluated weight, BMI, and fat mass (33-38), five studies evaluated blood pressure (33,35-38), six studies evaluated LDL (33,34,36-38), four studies evaluated heart rate (33,35,37,38), four studies evaluated waist circumference (34,35,37-38), five studies evaluated total cholesterol and triglycerides (33,34,36-38), four studies evaluated HDL (33,36-38)(33, 36 – 38), and only one study evaluated glucose 37.
Table 1. Characteristics of included studies.
|
First author, year |
Design |
Study population |
Intervention |
Control |
Intervention duration |
|---|---|---|---|---|---|
|
Hoddy K, 2014 |
A randomized controlled trial using a stratified sample. |
Sample of 74 obese subjects, aged between 25 and 65 years. ADF-L: 20 participants; 45 ± 3 years. ADF–SM: 20 participants, 46 ± 2 years. ADF-D: 19 participants, 45 ± 3 years. Chicago, E.E.U.U. |
Alternate day fasting (ADF): All subjects consumed 25% of their initial energy requirements on the fasting day (24 h) and ate ad libitum on each alternate feeding day (24 h). 1) ADF-lunch (ADF-L), 2) ADF-dinner (ADF-D), o 3) ADF-small meal (ADF-SM) |
Each subject who participated in a 2-week baseline period was asked to maintain stable body weight and continue their usual diet. |
10 weeks |
|
Bhutani S, 2010 |
A randomized controlled trial. |
Sample of 16 obese subjects, intervention group 45 to 51 years old. Intervention group Women 45 ± 3 years old / Men 46 ± 5 years old. women 75% y men 25%. Chicago, E.E.U.U. |
Alternate day fasting (ADF): ADF controlled feeding phase: Each subject participated in a 4-week ADF controlled feeding period, where their feeding was administered. Self-selected ADF feeding phase: subjects underwent a 4-week self-selected ADF feeding period, but their feeding was not administered. All subjects consumed 25% of their initial energy requirements on the fasting day and ate ad libitum on the feeding day. |
Initial 2-week control phase, each subject was required to maintain their body weight stable by maintaining their usual eating and activity habits. |
10 weeks |
|
De Oliveira I, 2021 |
A randomized controlled trial. |
Sample of 56 obese women, age between 19 to 44 years in both groups. women 100%. Control age: 31.03 ± 7.16 years old. Intervention age: 31.80 ± 6.96 years old. Maceió-Alagoas, Brasil. |
Time-restricted fasting (TRF) Hypoenergetic diet (with a deficit of 500 - 1000 kcal of energy expenditure). HCO: 45-55%; Lip: 25-30% Prot: 15-25%. Time restriction for the feeding period. 12-hour fast and 12-hour feeding window. |
Hypoenergetic diet (with a deficit of 500-1000 kcal of energy expenditure) HCO: 45-55%; Lip: 25-30% Prot: 15-25%. |
48 weeks |
|
Varady K, 2009 |
A randomized controlled trial. |
Sample of 16 obese subjects, 12 women and 4 men, the same used in the control and intervention. Age between 35 – 65 years old. Chicago, E.E.U.U |
Alternate day fasting (ADF): 4-week weight loss phase / ADF controlled food intake phase: All subjects consumed 25% of their baseline energy requirements on the "fast" day (24 h) and then consumed food ad libitum on each alternate "feed" day (24 h), providing food to the subjects. 4-Week Weight Loss/ADF Self-Selected Food Intake Phase: Subjects still consumed 25% of their baseline energy requirements on the fasting day and consumed food ad libitum on the day of feeding. However, during this period, no food was provided to the subjects. |
Subjects were asked to maintain stable body weight by maintaining their usual eating and exercise habits for 2 weeks. As such, each subject served as their own control. |
10 weeks |
|
Bhutani S, 2013 |
A parallel-arm randomized controlled trial. |
Sample of 83 obese participants, age between 25 to 65 years old. ADF intervention: 25 participants; 42 ± 2 years old. Exercise intervention: 24 participants; 42 ± 2 years old. Combined intervention: 18 participants; 45 ± 5 years old. Control: 16 participants; 49 ± 2 years old. Chicago, E.E.U.U |
Combination group and ADF group: 4-week controlled feeding period (they consumed 25% of their initial energy requirements on the "fasting day" and consumed food ad libitum on each "feeding day") and a 8-week self-selected feeding period. (they continued the ADF regimen but no food was provided for the fast day). |
Subjects in the control and exercise groups were asked to maintain their usual eating habits and were not provided with any alimentary or dietary advice. Subjects in the control and exercise group consumed an ad libitum amount of food each day. |
12 weeks |
|
Klempel M, 2012 |
A randomized controlled trial. |
Sample of 32 obese participants, between 25-65 years old. ADF – HF: 15 participants; 42,4 ± 3 years old ADF – LF: 17 participants; 43,2 ± 2,3 years old Chicago, E.E.U.U |
For 8 weeks: ADF-HF (100 g total fat) or ADF-LF (55 g total fat) intervention. All subjects consumed 25% of their energy requirements on the fasting day (24-hour period) and 125% of their energy requirements on the feeding day (24-hour period). The same macronutrient composition was used. |
For 2 weeks, each subject participated in an initial 2-week weight maintenance period in which they consumed either the HF (100 g total fat) or LF (55 g total fat) diet, providing 100% of their energy requirements. The same macronutrient composition was used. |
10 weeks |
3.3 Risk of bias assessment
The risk of bias assessment is presented in Figure 2.
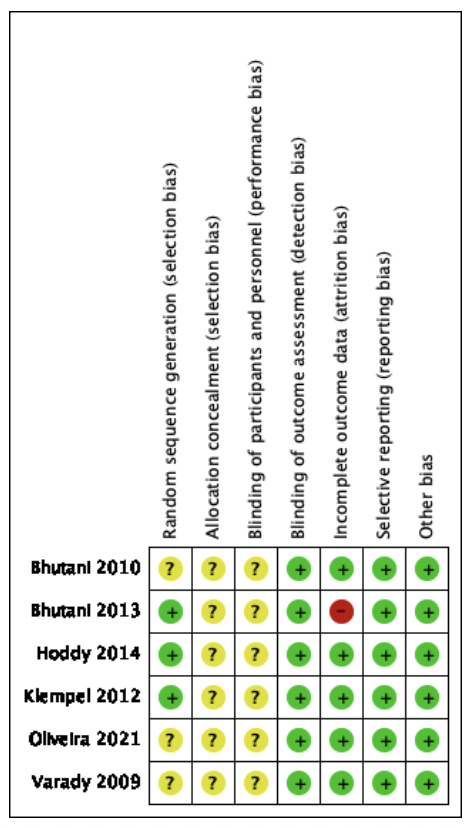
Green: low risk of bias; yellow: unclear risk of bias; and red: high risk of bias.
Of the 6 studies included in the SR (see figure 2), 3 studies presented a low risk of selection bias (33, 37, 38)because they reported details of their random sequence generation. Three studies, Bhutani et al., De Oliveira et al., and Varady et al. (34-36) presented an unclear risk of selection bias since it does not indicate how the study participants were selected. All 6 studies did not clearly state a method of allocation concealment, thus having an unclear risk of bias.
All studies assessed are at unclear risk of conduct or performance bias as they do not state whether the administrator remains masked.
Regarding the risk of Detection bias, all the studies included in this systematic review have a low risk of bias, although the study is open, the treatment administrators cannot interfere in the measurement of the response variable, because automated instruments were used.
Regarding attrition or follow-up bias, five studies presented a low risk, while Bhutani et al.37 had a high risk of bias, as there is a large difference in the number and gender of participants in each intervention.
In reporting bias, all studies were at low risk of bias, as significant study findings were reported.
Results of included studies
The main results of the included studies are summarized in Table 2. Of the 6 papers used in the systematic review, statistically significant differences were found in weight loss and BMI (Table 2-A) at the end of the interventions, in the case of Hoddy et al. 33, Bhutani et al. 34, Varady et al. 36, Klempel et al. 38 with 10 weeks of intervention and Bhutani et al. 37 with 12 weeks of intervention, reducing body weight in a range of 3 to 6 kg and BMI in a range of approximately 1 to 3 kg/m2. However, De Oliveira et al. 35 did not present significant changes after 48 weeks of intervention in both variables.
Table 2A. Results of included studies .
|
First author, year |
Weight (kg) |
BMI (kg/m2) |
Fat mass (kg) |
LDL (mg/dl) |
BP (mmHg) |
Glucose (mg/dl) |
|---|---|---|---|---|---|---|
|
Hoddy K, 2014 |
Effect size (pre-post): ADF–L: 3,5±0,4 (P <0,001) ADF–D: 4,1±0,5 (P <0,001) ADF–SM: 4,0±0,5 (P <0,001)
|
Effect size (pre-post): ADF-L: 1.3±0.2 (P <0,050) ADF-D: 1.4±0.2 (P <0,050) ADF-SM: 1.5±0.2 (P <0,050)
|
Effect size (pre-post): ADF-L: 0,075±0,027 (P <0,001) ADF-D: 0,135±0,042 (P <0,001) ADF-SM: 0,135±0,032 (weekly) (P <0,001) |
ADF – L: Pre:114±11 Post: 113±9 (P=0,540) ADF – D: Pre:120±6 Post: 119±8 (P= 0,980) ADF–SM: Pre:110±7 Post:110±7 (P= 0,790)
|
SBP: ADF – L: Pre:115±3 Post: 112±3 (P=0,570) ADF – D: Pre:117±3 Post: 112±2 (P=0,070) ADF – SM: Pre:119±3 Post: 112±3 (P=0,040)
DBP: ADF – L: Pre:78±2 Post: 76±2 (P=0,560) ADF – D: Pre: 79±2 Post: 76±1 (P=0,150) ADF – SM: Pre: 83±1 Post: 79±1 (P=0,600)
|
|
|
Bhutani S, 2010 |
Pre: 96±5,3 kg CFI (day 42): Post: 93,7±5 (P <0,050) SFI (day 70) Post: 90,8±4,8 (P <0,050) |
Pre: 33,7±1 CFI (day 42) Post: 32,8±0,9 (P <0,050) SFI (day 70) Post: 31,4±0.9 (P <0,050) |
Pre: 43±2,2 CFI (day 42) Post: 41,3±2,7 (P <0,050) SFI (day 70) Post: 38,1±1,8 (P <0,050) |
Pre: 106±10 CFI (day 42) Post: 73±9 (P <0,050) SFI (day 70) Post: 72±8 (P <0,050) |
|
|
|
De Oliveira I, 2021 |
HD: Pre: 80,25±9,4 Post: 77,53 CI95% [71,99; 83,07] HD+TRF: Pre: 81,25±13,51 Post: 87,33 CI95% [76,81; 97,84]* (P=0,960)
|
HD: Pre: 33,12±3,63 Post: 77,53 CI95% [71,99; 83,07] HD+TRF: Pre: 33,53±4,53 Post: 87,33 CI95% [76,81; 97,84]* (P=0,840)
|
HD: Pre: 43,55±4,7% Post: 44,24 CI95% [41,13; 47,35]
HD+TRF: Pre: 44,41±5,47% Post: 46,21 CI95% [42,69;49,74]* (P=0,020) |
|
SBP: HD: Pre:124,03±11,28 Post: 119,44 CI95% [115,47;123,41] HD+TRF: Pre: 127,1±15,29 Post: 122,41 CI95% [116,49;128,34]* (P=0,480)
DBP: HD: Pre: 86,51±10,08 Post: 82,29 CI95% [78,05;86,54] HD+TRF: Pre: 86,2±13,2 Post: 83,8 CI95% [79,55;88,05]* (P=0,460) |
|
|
Varady K, 2009 |
Effect size (pre-post): CFI – ADF: 0,67±0,1 (week) SFI – ADF: 0,68±0,1 (week) (P <0,001)
|
Pre: 33,7±1,0 CFI-ADF: Post: 32,8±1,0 (P <0,001) SFI-ADF: Post: 29,9±2,1 (P <0,010) |
Effect size (post – pre): 5,4 ± 0,8 kg (P <0,010) |
Pre: 102±9 CFI-ADF: Post: 73±9 SFI-ADF: Post: 72±8 (P <0,010) |
Effect size (pre-post): SBP: CFI – ADF: 4,4±1,8% SFI – ADF: 5,1±1,6% (P <0,050)
DBP: Pre: 80,3 ± 2,7
CFI-ADF: Post: 79,2±2,1 SFI-ADF: Post: 78,8±2,5 NS |
|
|
Bhutani S, 2013 |
Combination: Pre: 91±6 Post: 85±6 (p<0,001)
ADF: Pre: 94±3 Post: 91±3 (p<0,001)
Exercise: Pre: 93±2 Post: 92±2 (p=0,027)
Control: Pre: 93±5 Post: 93±5 (p=0,577)
|
Combination: Pre: 35±1 Post: 33±1 (p<0,001)
ADF: Pre: 35±1 Post: 34±1 (p<0,001)
Exercise: Pre: 93±2 Post: 34±1 (p=0,030)
Control: Pre: 93±5 Post: 35±1 (p=0,707)
|
Combination: Pre: 45±2 Post: 40±2 (p<0,001)
ADF: Pre: 43±2 Post: 41±2 (p=0,008)
Exercise: Pre: 46±2 Post: 45±2 (p=0,182)
Control: Pre: 43±4 Post: 43±4 (p=0,570) |
Combination: Pre: 125±9 Post: 109±11 (p=0,043)
ADF: Pre: 113±8 Post: 112±9 (p=0,917)
Exercise: Pre: 113±5 Post: 113±7 (p=0,947)
Control: Pre: 119±6 Post: 123±8 (p=0,586)
|
SBP: Combination: Pre: 113±3 Post: 111±3 (p=0,262) ADF: Pre: 124±3 Post: 120±3 (p=0,007)
Exercise: Pre: 113±2 Post: 115±3 (p=0,284)
Control: Pre: 122±5 Post: 129±6 (p=0,603)
DBP: Combination: Pre: 76±2 Post: 76±2 (p=0,939) ADF: Pre: 82±2 Post: 80±2 (p=0,034) Exercise: Pre: 71±2 Post: 76±2 (p=0,976) Control: Pre: 76±3 Post: 84±4 (p=0,480) |
Combination: Pre: 94±2 Post: 92±3 (p=0,589) ADF: Pre: 98±5 Post: 95±5 (p=0,146)
Exercise: Pre: 92±2 Post: 91±2 (p=0,862)
Control: Pre: 109±7 Post: 111±6 (p=0,637)
|
|
Klempel M, 2012 |
Effect size (pre-post): ADF-HF: 4.3 ± 1.0 ADF-LF: 3.7 ± 0,7 (P <0,0001)
|
Effect size (pre-post): ADF-HF: 1,7 ± 0,4 ADF-LF: 1,5 ± 0,3 (P <0,0001)
|
ADF-HF: Pre: 43,7±1,9 Post: 38,3±1,8
ADF-LF: Pre: 43,6±1,8 Post:39,4±39,4 (P <0,0001)
|
ADF-HF: Pre: 119±10 Post: 90 ± 7
ADF-LF: Pre: 124±6 Post: 85 ± 7 (P <0,0001)
|
SBP: ADF-HF: Pre: 111±2 Post: 109±2
ADF-LF: Pre: 116±3 Post: 118±3 NS
DBP: ADF-HF: Pre: 77±3 Post: 75±2
ADF-LF: Pre: 75±2 Post: 81±3 NS |
Table 2B. Results of included studies.
|
First author, year |
HR (bpm) |
WC (cm) |
TC (mg/dl) |
TG (mg/dl) |
HDL (mg/dl) |
|---|---|---|---|---|---|
|
Hoddy K, 2014 |
ADF – L: Pre: 69±3 Post: 64±2 (P<0,001) ADF – D: Pre: 71±2 Post: 68±2 (P=0,050) ADF – SM: Pre: 72±3 Post: 72±3 (P=0,800) |
|
ADF – L: Pre: 190±11 Post: 184±9 (P=0,920) ADF – D: Pre: 199±8 Post: 190±8 (P=0,190) ADF – SM: Pre:185±8 Post: 181±7 (P=0,820) |
ADF – L: Pre: 104±15 Post: 96±14 (P=0,380) ADF – D: Pre: 108±14 Post: 101±12 (P=0,100) ADF – SM: Pre: 94±9 Post: 84±9 (P=0,910) |
ADF – L: Pre: 55±3 Post: 52±3 (P=0,220) ADF – D: Pre: 57±4 Post: 54±3 (P=0,700) ADF – SM: Pre: 57±3 Post: 55±3 (P=0,690) |
|
Bhutani S, 2010 |
|
Pre: 109±2 CFI (day 42) Post: 106±3 (P <0,050) SFI (day 70): Post: 105±3 (P <0,050) |
Pre: 175 ± 8 CFI (day 42) Post: 140±7 (P <0,050) SFI (day 70): Post: 138±8 (P <0,050) |
Pre: 136±17 CFI (day 42) Post: 110±17 (P <0,050) SFI (day 70): Post: 88±15 (P <0,050) |
|
|
Oliveira I, 2021 |
HD: Pre: 71,85±9,48 Post: 73,74 CI95% [69,62;77,85] HD+TRF: Pre: 76,27±8,79 Post: 74,35 CI95% [71,79;76,91]* (P=0,110) |
HD: Pre: 98,86±9,61 Post: 97,07 CI95% [91,15; 102,98] HD+TRF: Pre: 102,79±3,27 Post: 103,84 CI95% [95,32; 112,36]* (P=0,100) |
|
|
|
|
Varady K, 2009 |
Significantly reduced (P < 0.050) It does not report the values, but it does report the trend in a figure. |
|
Pre: 175±8 CFI-ADF: Post: 140±7 SFI-ADF: Post: 138±8 (P <0,001) |
Pre: 125±15 CFI-ADF: Post: 110±17 SFI-ADF: Post: 88±15 (P <0,010) |
Pre: 48±4 CFI-ADF: Post: 45±4 SFI-ADF: Post: 46±3 NS |
|
Bhutani S, 2013 |
Combination: Pre: 78±2 Post: 76±2 (p=0,384) ADF: Pre: 75±2 Post: 75±2 (p=0,711) Exercise: Pre: 71±2 Post: 71±2 (p=0,925) Control: Pre: 76±3 Post: 77±3 (p=0,763) |
Combination: Pre: 91±2 Post: 88±1 (p<0,001) ADF: Pre: 100±2 Post: 95±2 (p<0,001) Exercise: Pre: 98±2 Post: 95±2 (p<0,001) Control: Pre: 99±3 Post: 97±2 (p=0,640) |
Combination: Pre: 190±10 Post: 186±12 (p=0,658) ADF: Pre: 171±8 Post: 183±11 (p=0,053) Exercise: Pre: 181±6 Post: 181±8 (p=0,921) Control: Pre: 185±7 Post: 187±10 (p=0,784) |
Combination: Pre: 77±7 Post: 87±8 (p=0,161) ADF: Pre: 81±7 Post: 86±8 (p=0,341) Exercise: Pre: 74±6 Post: 79±5 (p=0,290) Control: Pre: 97±13 Post: 102±11 (p=0,452) |
Combination: Pre: 50±3 Post: 59±4 (p=0,041) ADF: Pre: 49±2 Post: 49±3 (p=0,807) Exercise: Pre: 51±2 Post: 52±3 (p=0,457) Control: Pre: 52±3 Post: 56±3 (p=0,166) |
|
Klempel M, 2012 |
ADF-HF: Pre: 75±3 Post: 77 ± 3 ADF-LF: Pre: 76±3 Post: 73 ± 2 NS |
ADF-HF: Pre: 98±1,8 Post: 91±1 ADF-LF: Pre: 98,8±2.1 Post: 91,5±2.1 (P <0,001) |
ADF-HF: Pre: 206±11 Post: 172±9 ADF-LF: Pre: 201±6 Post: 162±7 (P <0,0001) |
ADF-HF: Pre: 125±17 Post: 108±15 ADF-LF: Pre: 108±13 Post: 83±10 (P <0,001) |
ADF-HF: Pre: 63±5 Post: 108±15 ADF-LF: Pre: 60±4 Post: 83±10 NS |
abbreviations: ADF-L, alternate day fasting-lunch; ADF-D, alternate day fasting-dinner; ADF-SM,
alternate day fasting-small meals; BMI, body mass index; LDL, low-density lipoprotein; BP, blood
pressure; HR, heart rate; WC, waist circumference; TG, triglycerides; CFI, controlled food intake;
SFI, self-selected food intake; BFP: body fat percentage; SBP; systolic blood pressure; DBP:
diastolic blood pressure; bpm, beats per minute.
All data ± SEM. *95% confidence interval.
In the 6 included studies (33-38), statistically significant differences were found for the variable of fat mass (Table 2A), with a duration of interventions ranging from 10 to 48 weeks, decreasing approximately 1.3 to 5.4 kg.
In the case of the waist circumference variable (Table 2B), the studies showed that there is a significant difference in the studies by Bhutani et al. (34), Klempel et al. (38), and Bhutani et al. (37), with a range of 10 to 12 weeks of intervention and a decrease of 4 to 7 cm. The case of De Oliveira et al. (35) did not present significant changes for this variable.
Regarding the lipid profile (Table 2B), significant changes were observed for total cholesterol, LDL cholesterol, and triglycerides in the studies by Bhutani et al. (34), Varady et al. (36), and Klempel et al. (38), which have a duration of 10 weeks. On the other hand, Hoddy et al. (33), and Bhutani et al. (37), did not present statistically significant changes in interventions of 10 and 12 weeks, respectively. De Oliveira et al. (38) do not present results for these variables.
Only 4 studies evaluated HDL cholesterol (Table 2B), Hoddy et al. (33), Varady et al. (36), Bhutani 20 et al. 13 (37), and Klempel et al. (38), of which, they did not present significant changes for the variable, varying between 10 and 12 weeks of intervention. De Oliveira et al. (35) did not present results.
The glucose variable was only studied by Bhutani et al. (37), with 12 weeks of intervention in which no statistically significant difference was generated (Table 2A).
Regarding heart rate (Table 2A), it was analyzed in 5 studies, presenting a significant difference in the study by Varady et al. (36), and Hoddy et al. (33). Both studies were developed in a time of 10 weeks. The latter only showed a significant difference in ADF-L (fasting on alternate days at lunch), marking a difference between pre and post-study of 6 beats per minute, but not for ADF-D and ADF-SM.
Of the 6 included studies, significant differences in SBP were only found in the study by Varady et al. (36), in which it was significantly reduced after completing the phase of controlled and self-selected food intake, during 10 weeks of intervention Table 2A).
Results of meta-analyses
Only 2 studies were available (34,36) about five variables: BMI, fat mass, total cholesterol, LDL cholesterol, and triglycerides. Both trials have the same intervention time of 10 weeks. Alternate day fasting was found to cause a significant change in BMI (WMD = -3.93, 95% CI -5.01, -2.85 kg/m2). Regarding the effect sizes of intermittent fasting on fat mass, in the same two studies (34,36), with the same intervention time, statistically significant observed mean effect sizes were found (WMD = -5.12, 95 % CI -6.16, -4.08 kg). Regarding the lipid profile, for total cholesterol, a WMD = -37,00 (CI 95%: -40,91; -33,01 mg/dl), and for LDL a WMD = -31,88 (CI 95%: -36,17; -27,58 mg/dl). For triglycerides, the change was also statistically significant. (WMD = -42,32, CI 95%: -53,09; -31,55 mg/dl). Details are available in Table 3.
Table 3. Meta-analysis results .
|
Response Variable |
First author and year |
Intervention sample |
Control sample |
Weight (%) |
Mean difference |
CI 95% |
Heterogeneity |
||
|---|---|---|---|---|---|---|---|---|---|
|
I2 |
Q |
P-value |
|||||||
|
Intervention: Alternate Day Fasting (ADF) |
|||||||||
|
IMC (kg/m2) |
Buthani 2010 |
16 |
16 |
9,50 |
-5,20 |
-8,71; -16,9 |
|
|
|
|
Varady 2009 |
16 |
16 |
90,50 |
-3,80 |
-4,94; -2,66 |
|
|
|
|
|
WMD * |
32 |
32 |
100,00 |
-3,93 |
-5,01; -2,85 |
0 |
0,5536 |
0,4569 |
|
|
Masa grasa (kg) |
Buthani 2010 |
16 |
16 |
55,94 |
-4,90 |
-6,29; -3,51 |
|
|
|
|
Varady 2009 |
16 |
16 |
44,06 |
-5,40 |
-6,97; -3,83 |
|
|
|
|
|
WMD * |
32 |
32 |
100,00 |
-5,12 |
-6,16; -4,08 |
0 |
0,2185 |
0,6402 |
|
|
c-LDL (mg/dl) |
Buthani 2010 |
16 |
16 |
46,94 |
-34,00 |
-40,27; -27,73 |
|
|
|
|
Varady 2009 |
16 |
16 |
52,06 |
-30,00 |
-35,90; -24,10 |
|
|
|
|
|
WMD * |
32 |
32 |
100,00 |
-31,88 |
-36,17; -27,58 |
0 |
0,8290 |
0,3626 |
|
|
c-Total (mg/dl) |
Buthani 2010 |
16 |
16 |
48,37 |
-48,00 |
-59,09; -36,91 |
|
|
|
|
Varady 2009 |
16 |
16 |
51,63 |
-37,00 |
-47,39; -26,61 |
|
|
|
|
|
WMD * |
32 |
32 |
100,00 |
-37,00 |
-40,91; -33,01 |
0 |
0 |
1,000 |
|
|
Triglicéridos (mg/dl) |
Buthani 2010 |
16 |
16 |
48,37 |
-48,00 |
-59,09; -36,91 |
|
|
|
|
Varady 2009 |
16 |
16 |
51,63 |
-37,00 |
-47,39; -26,61 |
|
|
|
|
|
WMD * |
32 |
32 |
100,00 |
-42,32 |
-53,09; -31,55 |
50,31 |
2,0125 |
0,1560 |
|
The weight variable could not be subjected to meta-analysis because the comparison results are different, expressing the results in different ways, where Buthani et al. (34) express the initial and final weight of the study in total kg, whereas Varady et al. (36) present weight change in kg/week, so the authors of this study decided not to include it.
DISCUSSION
The results of our systematic review and meta-analysis aimed to determine the effectiveness of intermittent fasting on biochemical and anthropometric markers in obese adults with cardiovascular risk, in an age range of 18 to 65 years. In this context, for the weight and BMI variables, we found favorable changes in intermittent fasting interventions on alternate days (ADF) (33,34,36-38), between 10 and 12 weeks. The applied meta-analyses raised a statistically significant difference. This can be explained by the use of fat mass as an energy substrate in the fasting period, since normally the energy substrate used by the tissues corresponds to carbohydrates, provided through food or reserves. Due to the depletion of these deposits, the metabolic routes towards lipolysis are derived, with the obtaining of free fatty acids as an energy source and the decrease in metabolic energy expenditure as a result of physiological fasting, in this way the body weight decreases, and therefore the BMI. However, the study of De Oliveira et al. (35) published an experience with time-restricted feeding (TRF), with a duration of 48 weeks, where there were no differences at the end of the intervention. This may be due to the prolonged time of the study, where the adherence of the participants is diminished during the months without follow-up by a nutritionist, reflected in the anthropometric and biochemical results of the clinical trial. The study showed that by not having a strictly controlled environment, it did not cause adherence and maintenance of weight loss throughout the study in the participants.
In the case of fat mass in ADF interventions, our systematic review presents a positive change in the studies, with a decrease in kg (33,34,36-38), and is consistent with the meta-analysis carried out, and also for waist circumference (WC) (34,37,38). These data suggest that the weight loss observed with ADF is due to a decrease in fat mass and not fat-free mass (34), decreasing the resting metabolic rate, which allows for a greater capacity for energy utilization (37).
For the variables total cholesterol, LDL, and TG, there was a decrease in these variables with ADF (34,36,38), which had an intervention time of 10 weeks, as stated in the meta-analysis carried out. Circulating free fatty acid (FFA) oxidation increases during periods of weight loss, while FFA synthesis decreases (39, 40). A relationship is also observed between the increase in adiponectin concentrations and the decrease in LDL cholesterol and TG levels after treatment, where it is suggested that adiponectin can decrease the supply of FFA to the liver for gluconeogenesis, thus decreasing the synthesis of TG. This decrease results in attenuated VLDL secretion, which in turn would decrease plasma LDL cholesterol concentrations (40).
For the variable HDL, there were no changes in the studies (33,36-38). Evidence suggests that HDL cholesterol may require more than 16 weeks to make a change (38), as concentrations generally increase with exercise depending on intensity, thus requiring a controlled environment under supervision when combining ADF with exercise (41, 42). For this reason, it cannot be categorically confirmed that an increase occurs following an intermittent fasting protocol. More research should be done in this regard in future studies (43).
Heart rate was analyzed in five studies, showing a decrease in beats per minute in only two (33,36). This may be caused by reduced markers of systemic inflammation and oxidative stress associated with atherosclerosis, increasing heart rate variability by improving parasympathetic tone (44), although as described in other studies the findings are also somewhat surprising since ADF does not generally improve heart rate (37,38). Similarly, systolic blood pressure only had favorable changes in one study (36). This may be caused by weight loss and changes in eating habits (45,46). The above parameters improve when the intermittent fasting diet is combined with a physical exercise program in patients with obesity (37,46).
From a global standpoint, we can mention that the variables mentioned above are affected since fasting induces the coordinated alteration of metabolic and transcriptional mechanisms. After 12 to 36 hours of fasting, the human body enters a physiological state of ketosis characterized by low blood glucose levels, decreased liver glycogen deposition, and hepatic production of ketone bodies 48.
The production of ketone bodies generates oxidation of fatty acids as an energy source, which can be seen reflected in the decrease in biochemical and anthropometric parameters 49.
This study was not without limitations. The first accounts for the small number of studies that we were able to compile, therefore the evidence is not very robust to reach a reliable result, however, these studies are clinical trials that obey the best quality of evidence available at the time. In the same way, we wanted to meta-analyze in order to take advantage of the little evidence available and demonstrate a quantitative result, to reinforce the qualitative evidence of our review. Furthermore, the study by Davey et al. 50 shows that the number of studies eligible for meta-analyses is usually very small for all medical areas, outcomes, and interventions covered by Cochrane reviews. Due to the above, it is possible to consider opening other designs, as well as replicating this search for the next five years to evaluate if there are more clinical trials that meet the criteria and enter the second version of this SR.
Intra-study sample size with few participants between 16 and 83 people is also observed, so the evidence may overestimate the results obtained, however, the studies included in this systematic review are characterized by presenting a control in the procedures performed that require interventions. Therefore, it is recommended to carry out studies increasing the sample size.
In the same way, some studies can be observed with an intervention time of 1 year without achieving favorable changes, due to poor adherence to treatment, directly affecting the response variables. The other studies included an intervention time of 10 to 12 weeks and presented better adherence to treatment. Therefore, it is recommended to have constant monitoring during the intervention time, favoring the adherence of the participants to obtain positive results.
We found few long-term studies, therefore the long-term results of this type of intervention are unknown. However, studies of shorter duration had a positive impact on the variables. Therefore, the effectiveness of intermittent fasting for prolonged periods cannot be verified. Our recommendation for possible future studies is to determine the long-term effectiveness.
No study presented adverse effects in the participants assigned to the comparison groups, therefore, they do not report side effects in themselves, which is a lack of evidence, limiting the ability to analyze the results. That is why we propose to show the adverse effects, to verify the safety and integrity of the participants in this type of treatment in future studies.
It was only possible to perform the meta-analysis of 2 studies with a sample size of 16 participants in each one, so the statistical power is low, although according to Davey et al. 50, the minimum number of studies needed to be eligible for a meta-analysis is two studies and only two individuals. The studies included in this review are of high quality and with a moderate to low degree of heterogeneity, with low variability between studies.
Regardless of the limitations mentioned, it is expected that in future studies of this nature long-term interventions will be carried out with a greater number of participants, increasing adherence to the ADF diet, hoping for better results. However, the results found are relevant, since studies of the highest quality were reviewed, because clinical trials were included, showing relevant changes in interventions of 10 to 12 weeks, with favorable changes in biochemical and anthropometric parameters. With this systematic review, we show the current state of research regarding the efficacy of intermittent fasting in obese patients, therefore, researchers are encouraged to design more studies to verify with a higher degree of statistical power the results concerning the study hypothesis.
CONCLUSION
In conclusion, we can determine that there is evidence that allows us to think of a favorable relationship between intermittent fasting and the reduction of cardiovascular risk in obese patients, as a dietary therapeutic strategy for a possible nutritional approach, generating changes in different variables such as weight, BMI, fat mass, lipid profile, waist circumference, systolic blood pressure, and heart rate. Weight loss and the effect produced by fasting could help reduce fat, improving the lipid profile of the groups intervened for a set time, in which, studies with an intervention time of 10 to 12 weeks showed interesting changes.
Authorship contributions: FO-C participated in the conception of the article, data collection, writing, and approval of the final version. FG-Z participated in the conception of the article, data collection, writing, and approval of the final version. ML-E participated in the conception of the article, statistical data analysis, writing, and approval of the final version.
Funding sources: Self-financed.
Conflicts of interest: The authors declare no conflict of interest.
Received: april 18, 2022
Approved:September 12, 2022
Correspondence: Prof. Miguel López-Espinoza.
Address:High Performance Center Campus. N°1855 Circunvalación Poniente, Talca. Chile
.
Telephone number:+56942629804
E-mail:mlopez34@santotomas.cl
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
REFERENCES