ARTICULO ORIGINAL
DOI 10.25176/RFMH.v19.n1.1788CHARACTERISTICS OF CERVICAL-UTERINE CANCER SCREENING AT 08 HEALTH INSTITUTIONS, METROPOLITAN-LIMA 2017
CARACTERÍSTICAS DEL TAMIZAJE PARA CÁNCER CÉRVICO-UTERINO EN 08 ESTABLECIMIENTOS DE SALUD, LIMA METROPOLITANA 2017
Aleksandar Cvetkovic-Vega1,a,b,
Carlos León-Nakamura1,a,b
, Alejandro Yábar-Berrocal1,a,b
1 Facultad de Medicina Humana, Universidad Ricardo Palma, Lima - Perú.
2 Instituto de Investigación en Ciencias Biomédicas, Ricardo Palma University, Lima - Peru.
a Surgeon.
b Surgeon, Specialist in Gynecology-Obstetrics.
c Surgeon, Specialist in Pathological Anatomy.
ABSTRACT:
Introduction: Use of Papanicolaou’s test versus Liquid Based Cytology; professional that
executes the sample’s extraction,
first lecture and diagnosys; use of Bethesda clasification; VPH’s DNA detection, and the follow up of ASCUS
results, are
important topics of the Cervical Cancer screening which are scarcely studied at Peru.
Objective: To determinate characteristics
from the screening of Cervical Cancer at 08 Health institutions (HI) from Metropolitan-Lima in 2017 which
were Intership
Medical Centers for FAMURP students.
Methods: Observational, descriptive transversal study with convenience type sampling.
Pathology’s Chiefs from HI were interviewed.
Results: 8 Third level HI from MINSA, ESsalud and FFAA were included. All HI
use Papanicolaou Test, no LBC were used. Samples were taken by the Gynaecologist or the Obstetrician and in
one case by
the nurse; first lecture, by the Medical Tecnologyst and in one case by the anatomic pathology specialist;
diagnosys, by
the anatomic pathology specialist. The Bethesda System was used at the Final Report. No HPV- DNA detection
test was used.
8 HI made ASCUS finding follow up.
Conclusion: LBC, automatic lecture for screening and HPV-DNA detection are not implemented.
It is recommended to desing cost-effective studies for their future implementation, and desing studies at the
Cervicuterine cancer
Screening stages.
Key words: Papanicolaou Test; Liquid-based Cytology; Cervicuterine Cancer; HPV; Screening.
(source: MeSH NLM)
RESUMEN:
Introducción: El uso de Citología Convencional de Papanicoloau (CC) frente a Base Líquida
(CBL), el profesional
que realiza la extracción, primera lectura y diagnóstico de la muestra; el uso de la clasificación Bethesda,
la
detección de ADN del VPH y el seguimiento de hallazgos ASCUS son eslabones en el tamizaje del Cáncer del
Cuello
Uterino (CCU) poco estudiadas en nuestro medio.
Objetivo: Determinar las características del tamizaje para cáncer
CCU en 08 establecimientos de salud (EESS) de Lima Metropolitana sedes del Internado Médico de la FAMURP en
el 2017.
Métodos: Descriptivo, transversal. Muestreo por conveniencia. Se entrevistó a los Jefes de
los Servicios de Patología.
Resultados: Participaron 8 EESS de Nivel III del MINSA, EsSalud y FFAA. Todos realizaron la
CC, ninguno CBL. Las muestras
son extraídas por el Ginecólogo o la Obstetriz, y en 1 caso por enfermería, la primera lectura es realizada
por el
Tecnólogo Médico y en un caso por el Anátomo Patólogo; el diagnóstico, por el Anátomo Patólogo. Se usa el
Sistema de
Bethesda para el Reporte Final. No se usan pruebas de detección para ADN del VPH. En 8, se hace seguimiento
para
hallazgos ASCUS.
Conclusión: La CBL, la lectura automatizada, y la identificación del ADN de VPH no están
implementados.
Se recomienda realizar estudios costo-efectivo para proponer su implementación futura, y realizar estudios
respecto de
las fases del proceso del Tamizaje.
Palabras clave: Prueba de Papanicolaou; Citología; Cáncer Cérvico
Uterino; PVH; Tamizaje. (fuente: DeCS BIREME)
INTRODUCTION
Cervical-uterine cancer (CUC) is a disease mainly caused by the oncogenic subtypes 16 and 18 of the Human Papillomavirus (HPV)1, being the fourth most frequent cancer worldwide2. The incidence of CUC in countries varies per thousand, in Bolivia (36.4), Peru (34.5) in contrast to developed countries such as Canada (6.6) and the United States (USA) (5.7), with a clear pattern between the incidence and degree of development of the country3. In Peru it is the first cause of death from cancer in general4 and in women5 causing the loss of 378 thousand years of healthy life equivalent to 900 million dollars per year, establishing the CUC as one of the main causes of lost healthy years of life and economic loss6.
The cytopathic effect of HPV infection1 is evidenced by opportunity detection7 and ideally in asymptomatic stages8 of precancerous lesions that, according to international bodies such as the WHO, must be classified and reported through the Bethesda System3,5 updated to 20144,9, this responsibility falls to the Cytopathologist Medical Specialist10. This detection is done as part of a screening program that includes methods such as the detection of HPV DNA whose finding is definitive; Conventional Pap smear that despite being within reach of the first level of care in the country7 has intrinsic limitations that add to the problem of coverage and the health system per se11; and Liquid-based Cytology with established advantages such as reduction by 70-90% of the rate of unsatisfactory samples found with Conventional Cytology10, but whose implementation costs are apparently their main limitation.
The study of abnormalities in epithelial cytology performed as part of the CUC screening process includes stages involving sampling, first reading and final diagnosis; the first is considered as a fundamental process in which many studies identify errors at this level12 because of occasional takers13 with direct repercussions on the increase in the rate of false negatives. The first reading and final diagnosis includes the description of squamous cells and glandular cells, with special emphasis on the first group, corresponding to low-level or high-level intraepithelial squamous lesions degree, there being the possibility of a group of atypical characteristics of undetermined significance (ASCUS) and those in which they cannot be excluded the high-grade lesions (ASC-H)15. This ASCUS group is a group of interest for its potential malignancy16, and constitutes an inconclusive group reported by the Cytopathologist.
From all the above, it is evident that the type of screening, the use of the classification of Bethesda, the professional related to the taking, first reading and interpretation of the sample, the identification of HPV DNA and the follow-up of ASCUS findings are important characteristics of the screening process that must be described and subsequently studied in depth in our reality reason why, and given the scant scientific evidence reported in our country are the objective of the description in this study.
METHODS
Studio design
Observational, descriptive cross-sectional study.
Population and sample
Health Institutes of Metropolitan-Lima that were Official Headquarters for the Medical Internship of students of the Faculty of Medicine of the Ricardo Palma University during 2017. The sampling was for convenience, taking as analysis unit the Pathology Service of each Health Establishment represented through its respective Head of Department or its equivalent. As inclusion criteria they proposed (See Figure 1): 1) Health Institutes with Pathological and Gynecological Anatomy Service; exclusion criteria: 1) Pathological Anatomy Services that refer the samples to other health Institutes for reading sheets; 2) Pathological Anatomy Service whose data collection record is incomplete; and 3) Service that does not authorize the conduct of the investigation.
Variables
By reviewing the national and international literature and by expert opinion on the subject, the following variables were raised as representative in the screening process for CUC: Type of cytology (Conventional Cytology; Liquid Based Cytology), Professional who extracts the sample (Specialist Surgeon, Obstetrician, Nurse), Professional who performs the first reading (Pathologist Anatom, Medical Technologist, Automated), Professional who performs the diagnosis (Pathologist Anatom, Technologist Medical, Automated), Use of Bethesda’s Methodology for Final Reporting, Detection of HPV DNA, and Follow-up of cases with ASCUS findings.
Procedures
The tool used was a data collection form reviewed by the thematic advisers. Authorized interviews were conducted with the Heads of Pathology Service of the HI, The data collected were cleaned and sorted, being recorded in an Excel spreadsheet that was reviewed 03 times to avoid the error. The generated database was then processed by a statistical program for analysis.
Research ethics
International guidelines for the proper use of data and the preservation of ethics in research were followed. The protocol was approved by the Institutional Ethics and Research Committee of the Hospital Nacional Docente Madre Niño San Bartolomé. Subsequently, institutional authorization was obtained from the 08 Hospital Headquarters that accepted to participate in the study, agreeing to the subsequent publication of the results.
Data analysis
The data was processed with the statistical package Stataø 11.1 (Stata Corp. Texas, US) for Windows. Qualitative variables were analyzed using frequencies and percentages.
RESULTS
Among the general characteristics of the 8 Health Institutes (HI) participating in the study, it was obtained that according to segment 5 they belonged to the MINSA, 1 to EsSalud and 2 to the Armed Forces of Peru. All these establishments develop a level of Tertiary Care and are Level III-1, Level of Complexity 7. Additionally, 3 HI were located in the district of Jesús María, and the others in the districts of Cercado de Lima, El Agustino, San Juan de Miraflores, Pueblo Libre, Comas and Miraflores. (See Table 1).
Regarding the characteristics of cervical-uterine cancer screening, it was found that in all HI, there is no difference according to the segment; the Conventional Pap smear is performed, denying in all cases the use of Liquid Based Cytology. About the professionals who extract the sample, in 4 HI said samples are taken both by Medical Surgeons Specialists in Gynecology and/or by Graduates in Obstetrics, being the case that in only 1 HI of the MINSA Segment such work is assigned exclusively to Graduate Obstetrics. In an HI, the extraction is carried out by the Specialist Surgeon, the Licensed in Obstetrics and the Lic. in nursing. (Figure 1). Regarding the professional who performs the first reading, in 100% the HI is performed by the Medical Technologist, denying in all cases and in all segments that there is an automated system for such reading. As for the professional who makes the diagnosis, this work is in all cases in charge of the Pathologist Anatom. Regarding the Use of the Bethesda System for the Final Report, 100% of HI indicated using it in their report. No HI reported testing for HPV DNA in screening samples. Regarding the follow-up of ASCUS findings, this process was performed in 7 HI. (See Table 2)
Table 1. General characteristics of participating health Institutes.
|
N° % |
||
|
Segment |
||
|
MINSA |
5 |
62.5 |
|
EsSALUD |
1 |
12.5 |
|
Armed Forces of Peru |
2 |
25.0 |
|
Level of Attention |
||
|
Tertiary |
8 |
100 |
|
Category of HI |
||
|
Level III-1 |
8 |
100 |
|
Complexity Level |
||
|
VII |
8 |
100% |
|
District |
||
|
Cercado de Lima |
1 |
12.5 |
|
El Agustino |
1 |
12.5 |
|
San Juan de Miraflores |
1 |
12.5 |
|
Pueblo Libre |
1 |
12.5 |
|
Comas |
1 |
12.5 |
|
Jesús María |
2 |
25 |
|
Miraflores |
1 |
12.5 |
Table 2. Characteristics of cervical-uterine cancer screening in health institutes by segment.
|
SEGMENT (N=8) MINSA ESSALUD (N=5) (N=1) |
FFAA (N=2) |
||
|
Type of cytology |
|||
|
Conventional Pap smear Liquid Based Citology |
5 1 It is not performed in the HI |
2 |
|
|
Extracts the sample |
|||
|
Surgeon Licensed Obstetrics Licensed Nurse |
4 5 0 |
1 1 1 |
2 2 0 |
|
Performs the first reading |
|||
|
Anatom Pathologist Medical Technologist Automated |
0 1 5 1 It is not performed in the HI |
0 2 |
|
|
Makes the diagnosis |
|||
|
Anatom Pathologist |
5 |
1 |
2 |
|
Medical Technologist |
The professional doesn’t perform it |
|
|
|
Automated |
It is not performed in the HI |
|
|
|
Use of the Bethesda system for final reporting |
5 |
1 |
2 |
|
Detection of HPW DNA in the sample |
It is not performed in the HI |
|
|
|
Follow-up of ASCUS findings |
5 |
1 |
1 |

Figure 1. Selection Tree of Participating Health Establishments.
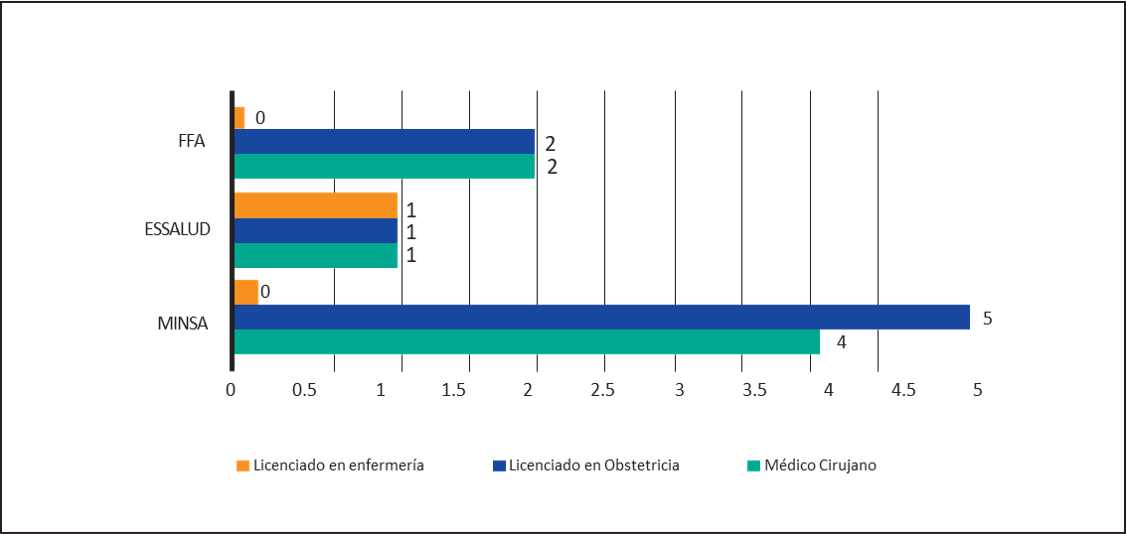
Graphic 1. Professional that extracts the sample from the screening
DISCUSSION
The CUC is the fourth most frequent cancer in the world2, generating in Latin American countries, viz Ecuador, a high cost effectiveness of $597 per year of life adjusted for disability17. In Peru, the estimated cost in 2011 generated by the CUC was $15.5 million18, being the first cause of death in women5 and therefore through the Ministry of Health, different strategies and policies have been implemented that highlight the importance of coverage, early detection and screening15, and it is with respect to the latter, that the findings regarding the Type of Cytology used, the professional that extracts, were studied in 8 HI, performs the first reading and gives the diagnosis, the use of Bethesda’s methodology in the Final Report, the use of HPV DNA detection and the follow-up of ASCUS findings.
It was found that in all HI (n=8) screening of CUC is performed by means of Conventional Pap smear (CC) and in no case by Liquid Based Cytology (LBC). This is consistent with findings from reports in developing countries in which CC is the most commonly used method6,19. However, despite its advantages and implementation, there are limitations in this technique that must be taken into account: starting with problems of coverage at national level18, very variable sensitivity and false negatives of up to 49%20,21 these last products of errors in the sampling, preparation or even interpretation; finally it is important to highlight the dependence of this method on the quality of the health system in the country in which it is implemented, to the point that studies indicate that in poorly organized systems, with few resources and little supply, the PAP is not useful in reducing the incidence of CUC22. That is why some studies report that the trend in the future is that screening programs use CBL4 which has a sensitivity of up to 80%, reducing both by 70 to 90% the rate of unsatisfactory samples found with CC20, as other CC problems previously described in literature23.
In addition, this technique allows the sample to be subjected to molecular DNA analysis of VPH24, allowing typing studies in addition, being considered by some studies as a viable and significantly better alternative in almost all aspects compared to CC25. However, the main disadvantage of this lies in the economic and implementation aspect. There are studies that propose that the CBL is an effective cost alternative considering the cost of a machine for CBL at $50,000 and the comparison between per capita input costs between the CC and the CBL, of $2.5 and $6 respectively26. This is based on the reduction in the number of false negatives, the amount of unsatisfactory samples, reduction in the time to obtain and evaluate the sample, the retention of the sample for further specialized studies27,28 and also, the reduction of the time to refer the patient for colposcopy and the time of follow-up and distancing in doing the following cytology when cytology and HPV are negative29.
The screening process for CUC can be considered in 5 stages11,30: two stages to be studied are the pre-analytic stage that covers the process of sample extraction which is indicated by some studies as the stage in which the majority of errors30 occurred. It may harm the other stages of the process11, and the post-analytical process to which 9% of errors30 are attributed. In our study, in the pre-analytical stage, the sample was extracted by the Specialist Surgeon in Gynecology and/or the Licensed in Obstetrics in 7 cases. In an HI, the latter carry out the extraction exclusively and in one in which the sample is taken by the Specialist Surgeon in Gynecology, the Graduates in Obstetrics and/or the Graduates in Nursing. Regardless of the professional who performs this process, it is important to highlight the expertise of the professional who performs it and the need for its continuous assessment by competencies through validated scales13, all this to counteract the problem of "casual takers" defined as personnel taking less than 50 samples per year13 and whose limitations in technique may contribute to inadequate samples giving a high rate of false negatives14.
In the analytical phase of screening, the interpretation of the result is performed. In our study it was evident that in 7 HI, the first reading of the sample was carried out by the Medical Technologist, with 1 HI in which he is in charge of both this and the Pathologist Anatom; and that in no case was there an automated system for the first reading. The literature mentions that the reading can be done by a cytologist, cytotechnologist or a qualified pathologist11. On the other hand, it is important to note that Automated Reading31 is not yet implemented in the HI, and to take into account the possible benefits that this would bring such as the reduction in 32% of the rate of false negatives32. There are studies that mention that, regardless of the type of cytology, with the use of Automated Reading the improvement in the performance and productivity of the process in terms of reading duration and number of samples analyzed per hour or day, was higher than manual33,34; however, there are also studies showing that despite higher productivity it is less effective cost compared to handreading33.
Regarding the professional who makes the diagnosis and also the use of the Bethesda System for the final report, in all the HI this function was in charge of the Specialist Anatom-Pathologist who in all cases used the aforementioned system; this is in line with international recommendations that indicate that10,11,30 allowing to operate a standardized language system, reasonably reproducible and flexible to the conditions16. However, this benefit must be accompanied by training and constant updating on the system aimed at both Pathological Anatomy Specialists and Gynecologists, in addition to overcoming the opinions of some authors regarding the impracticality of the Bethesda system both for the use of gynecological cytology learning and for the correlation of cytologic stages and findings30.
With regard to the use of HPV DNA testing in samples, it is described that no HI procedure is performed. There are established benefits with respect to this technique, which start from a simple extraction to the immediate start of the treatment once a positive result35 has been established. In addition, in some countries and specialized centers it has been proposed to use such targeted detection for high-risk HPV as a primary screening method16, achieving results of up to 60-70% of increased protection against CUC, being more effective than PAP and acetic acid test36, and extending the screening intervals up to 5 years in women with negative HPV37. The National Institute of Health, through its Bulletin of 2016, states that the molecular detection of HPV could be used before ASCUS type findings, however its implementation in a National Plan would involve high costs38.
Studies in Mexico indicate that strategies based on HPV DNA detection, virus typing, CBL and immunostaining as a triage procedure is a less expensive alternative compared to the per capita costs of treating cases with CIN 2-3 and CUC24. However, the unit cost is usually high and specialized logistics requirements are usually not accessible35, there are studies that classify it as highly cost-effective with $3482 AVAD39. Another potential benefit is the identification of the different HPV genotypes present in the Peruvian population, as suggested in some studies with unexpected findings for HPV-16 and other HPV-AR40; this would allow the development of a national registry and the evaluation of the impact of HPV vaccination strategies41. About this, they already exist in countries like Mexico studies such as the so-called FRIDA study whose objective is to evaluate the performance and cost-effectiveness of different triage alternatives in positive HPV women42.
Although the Bethesda System is recommended by the WHO, there are nonspecific alterations in epithelial cell samples called ASCUS that represent an intermediate point between benignity and malignancy, situation that has not been modified in the last Bethesda update of 2014; this is a group of important findings due to its malignancy potential43, a high frequency of reports in Conventional Cytology16, and whose follow-up should be mandatory. In contrast to ASCUS findings, the updated guidelines recommend the Co-Test that includes PAP with high-risk HPV DNA testing16. In this study, it was determined that 7 HI follow up on these findings, which indicates that not all HI have implemented strategies that highlight the importance of this process, despite being considered within the post analytical stage of screening for CUC11, nor considering that there are reports of lack of adherence rates close in some cases to more than 50%44.
As limitations, during the elaboration of the work, the lack of institutional policies in some HI to promote the development of multicenter work and the existence of policies that limit the development of research by researchers was identified outside their institutions. On the design and sampling side, the characteristics of the Study Universe prevented information from coming from other Hospital Headquarters in Metropolitan Lima whose results would have contributed to the enrichment of the study, Such is the case of the Centro de Prevención Larco, the Hospital Cayetano Heredial and the Hospital Arzobispo Loayza, for example. In addition, some HI perform the sampling and refer the result to their Network head establishments, which limits the description of the first process component of CUC screening in these establishments, and at the same time, the number of participants HI of the study decreases.
Recommendations
This preliminary study recommends the development of subsequent studies focused on the description of the phases of the screening process of the CUC and especially of the sampling; cost-utility studies of implementation of the CBL and the Co-test, and also in the implementation of automated methods of reading sheets. In addition, this study can be replicated by including a greater number of HI at regional and national level. The development of continuous trainings and evaluations through validated scales is recommended to the different professionals involved in the screening process, as well as joint training between Pathologist and Gynecologist Anatom Specialists on the Bethesda System updates and implementation.
Acknowledgements
The authors thank the collaboration of the Hospital Headquarters and their respective Heads of Service who agreed to participate in the study, to the Research Institute in Biomedical Sciences - INICIB - of the Faculty of Human Medicine of the Ricardo Palma University, and the Peruvian Student Medical Scientific Society-“SOCIMEP”.
Authorship contributions: The authors participated in the generation, collection of information, writing and final approval of the original article.
Financing: Self-financed.
Conflict of interest: The authors participated in the generation, collection of information, writing and final approval of the original article.
Received: November 19, 2018
Approved: December 20, 2018
Correspondence: Aleksandar Cvetkovic Vega
Address: Aleksandar Cvetkovic Vega.
Telephone: +51 964 982 676
Email: aleksandar.famurp@gmail.com
BIBLIOGRAPHICAL REFERENCES
-
1. Cox JT. Epidemiology and natural history of HPV. J Fam Pract. 2006 Nov;Suppl:3–9.
2. Globocan. Cervical Cáncer: Estimated incidence, mortality and prevalence worldwide in 2012 [Internet]. 2012 [cited 2016 Oct 27].
Available from: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp 3. Galán-Rodas E, Vélez CD, Rodas JL. Tamizaje citológico para cáncer de cuello uterino: una problemática emergente. Rev Cuerpo Méd Hosp Nac Almanzor Aguinaga Asenjo. 2013;6(2):52–4.
4. Santos-Ortiz C, Manrique J, Amorín E, Sarria G, Salazar M, Limache A, et al. Acelerando la innovación en el control del cáncer en el Perú. Rev Peru Med Exp Salud Pública. 2016 Aug 4;33(3):535.
5. Bruni L, Barrionuevo-Rosas L, Alberto G, Serrano B, Mena M, Gómez D, et al. Human Papillomavirus and Related Diseases in Peru. [Internet]. 2016 [cited 2016 Oct 27]. Available from: http://www.hpvcentre.net/statistics/reports/PER.pdf
6. Organización mundial de la salud. Perfiles oncológicos de los país: Perú [Internet]. Perú; 2014 [cited 2016 Oct 27]. Available from: http://www.who.int/cancer/countryprofiles/per_es.pdf?ua=1
7. Instituto Nacional de Salud. Acta de Acuerdos y Compromisos: Taller de Identificación de prioridades de investigación en salud 2015-2021 DISAS LIMA SUR y LIMA ESTE. [Internet]. Perú; 2014. Available from: http://www.ins.gob.pe/repositorioaps/0/2/jer/prior_reg/PRIORIDADES%20lima%20acta.pdf
8. Organización mundial de la salud. Papilomavirus humano ( PVH) y cáncer cervicouterino [Internet]. 2015. Available from: http://www.who.int/mediacentre/factsheets/fs380/es/
9. Zaharia M. El cáncer como problema de salud pública en el Perú. Rev Peru Med Exp Salud Pública. 2013;30(1):07–8.
10. Pelea CL. Nomenclatura de las lesiones cervicales (de Papanicolau a Bethesda 2001). Rev Esp Patol. 2003;36(1):5–10.
11. Arriaga VL, Gaspar DV. Control de calidad en Citología, Colposcopia y estudios Anatomopatológicos. Arch Méd Actual En [Internet]. 2012 [cited 2017 Feb 21]; Available from: http://www.medigraphic.com/pdfs/archivostgi/tgi-2012/tgi126g.pdf
12. Williamson SLH, Hair T, Wadehra V. The effects of different sampling techniques on smear quality and the diagnosis of cytological abnormalities in cervical screening. Cytopathology. 1997;8(3):188–95.
13. Sandra Olimpia Gutiérrez-Enríquez, Darío Gaytán-Hernández, José Manuel Zamarripa-Leyva, Yolanda Terán-Figueroa. Desempeño del personal de salud en la toma de las citologías cervicales: conocimientos teóricos y ejecución práctica [Internet]. Revista de Ginecología y Obstetricia de México; 2014. Available from: http://www.medigraphic.com/pdfs/ginobsmex/gom-2014/gom141c.pdf
14. Georgina O, Irma H. Análisis del descenso de la mortalidad por cáncer cervicouterino en el IMSS, 1991-2005. Rev Med Inst Mex Seguro Soc. 2006;44(Supl 1):S129–134.
15. Sarria-Bardales G, Limache-García A. Control del cáncer en el Perú: un abordaje integral para un problema de salud pública. Rev Peru Med Exp Salud Pública. 2013;30(1):93–8.
16. Nayar R, Wilbur DC. The Pap Test and Bethesda 2014. Acta Cytol. 2015 May 19;59(2):121–32.
17. Organización mundial de la salud, Organización panamericana de la salud. Directrices de la OPS/OMS sobre tamizaje y tratamiento de lesiones precancerosas para prevención del CCU.pdf. 2013.
18. Alfonso Gutiérrez-Aguado. Costo-utilidad de la vacuna contra el virus de papiloma humano en mujeres peruanas [Internet]. Revista Peruana de Medicina Experimental y Salud Pública; 2011 [cited 2016 Oct 27]. Available from: http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S1726-46342011000300003
19. Sharma J, Toi P, Siddaraju N, Sundareshan M, Habeebullah S. A comparative analysis of conventional and SurePath liquid-based cervicovaginal cytology: A study of 140 cases. J Cytol. 2016;33(2):80.
20. Berek JS, Novak E. Berek & Novak’s gynecology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012.
21. Santiago Alonso, Gema Vaquero, Rocío Montejo, Manuela López, Pilar Miranda, Monserrat López, et al. Comparación en el rendimiento diagnóstico de lesiones cervicales entre la citología convencional de Papanicolaou y la citología en base líquida, en una misma población de referencia. In Granada, España; 2006.
22. Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353(20):2158–68.
23. Ricci P, Perucca E, Koljanin J, Baeriswyl E. Citología de base líquida: revisión de la historia y los estudios al respecto. Rev Chil Obstet Ginecol. 2004;69(3):256–62.
24. Beal CM, Salmerón J, Flores YN, Torres L, Granados-García V, Dugan E, et al. Cost analysis of different cervical cancer screening strategies in Mexico. Salud Pública México. 2014;56(5):429–501.
25. Bak M, Séberné Éll M, Bóka M, Veleczki Z, Nyári T, Pete I, et al. Liquid-based cervical cytology ThinPrep screening in Hungary. Orv Hetil. 2014 May;155(18):708–14.
26. Cox JT. Liquid-based cytology: evaluation of effectiveness, cost-effectiveness, and application to present practice. J Natl Compr Canc Netw. 2004;2(6):597–611.
27. Guo J, Cremer M, Maza M, Alfaro K, Felix JC. Evaluation of a Low-Cost LiquidBased Pap Test in Rural El Salvador: A Split-Sample Study. J Low Genit Tract Dis. 2014 Apr;18(2):151–5.
28. Alameda F, Fusté P, Albert S, Romero E, Gimferrer E, Soler I, et al. Citología en medio líquido (Thin Prep Pap Test). Un año de experiencia. Prog Obstet Ginecol. 2007 Apr;50(4):197–202.
29. Olry de Labry Lima A, Epstein D, García Mochón L, Ruiz Aragón J, Espín Balbino J. Análisis de coste-efectividad de la prueba de citología cervicovaginal. Prog Obstet Ginecol. 2012 Aug;55(7):304–11.
30. Moya-Salazar J, Rojas-Zumaran V, Torres-Martínez R, Rosas-Vargas L. Calidad de los extendidos cervicouterinos dentro de la coloración de Papanicolaou para el cribado de cáncer cervical en Lima, Perú. Rev Esp Patol. 2016 Jan;49(1):7–18.
31. Rodríguez-Vázquez S. Support to the Diagnosis of the Pap Test, Using Computer Algorithms of Digital Image Processing. In: Sidorov G, Herrera-Alcántara O, editors. Advances in Computational Intelligence [Internet]. Cham: Springer International Publishing; 2017 [cited 2017 Sep 16]. p. 425–36. Available from: http://link.springer.com/10.1007/978-3-319-62434-1_35
32. Bansal B, Gupta P, Gupta N, Rajwanshi A, Suri V. Detecting uterine glandular lesions: Role of cervical cytology. CytoJournal. 2016;13(1):3.
33. De Tecnologías Sanitarias I de E. Métodos automatizados de lectura de citología cervical uterina. [cited 2016 Oct 29]; Available from: http://www.sergas.es/gal/publicaciones/docs/avalia-t/pdf-2236-ga.pdf
34. Eurocytology. Guías para la organización de laboratorio, procesamiento y screening [Internet]. [cited 2017 Feb 21]. Available from: http://www.eurocytology.eu/es/course/486
35. World Health Organization, World Health Organization, Reproductive Health and Research. Comprehensive cervical cancer control: a guide to essential practice. [Internet]. 2014 [cited 2016 Oct 27]. Available from: http://www.ncbi.nlm.nih.gov/ books/NBK269619/
36. Vargas-Hernández VM, Vargas-Aguilar VM, Tovar-Rodríguez JM. Detección primaria del cáncer cervicouterino. Cir Cir. 2015 Sep;83(5):448–53.
37. De Sanjosé S. Cambios en el cribado del cáncer de cuello uterino. Aten Primaria. 2016 Nov;48(9):563–4.
38. Hijar G. Detección molecular y genotipificación de virus del papiloma humano como tamizaje de cáncer de cuelo uterino: posibilidades en el contexto peruano. Bol-Inst Nac Salud. 2016;22(1-3):22–8.
39. Henríquez-Trujillo R, Narváez-Moscoso F. Estimación de la carga de enfermedad por cáncer de cuello uterino en Ecuador. Rev Médica. 2016;27(1):53–5.
40. Iwasaki Ricardo, Arias-Stella Javier Jr., Arias-Stella Javier. Prevalencia del virus de Papiloma Humano de alto riesgo en el Perú. Diagnóstico. 2014;53(1):5–8.
41. Marzo-Castillejo Merce B-BB, Vela-Vallespín Carmen, Nuin-Villanueva Marian, Bartolomé-Moreno Cruz, Melus-Palazón Elena, Vilarrubi-Estrella Merce. Recomendaciones de prevención de cáncer. Aten Primaria. 2016;48(1):39–59.
42. Group FS, others. Triage strategies in cervical cancer detection in Mexico: methods of the FRIDA Study. Salud Pública México. 2016;58(2):197–210.
43. Solom,D., Schiffman,M, Tarone, R. For the ASCUS/LSIL Triage Study for Cervical Cancer (ALTS) Group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance. Baseline results from a randomized trial. J Natl Cancer Inst. 2001;92(12):293–9.
44. Barrios L, Retamoso E, Alvis LR. Adherencia al seguimiento y evolución de la lesión en mujeres con Neoplasia intra epitelial cervical escamosa grado 1. Rev Colomb Cancerol. 2017 Jan;21(1):19–25.



