ORIGINAL PAPER
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2019 - Universidad Ricardo PalmaDOI 10.25176/RFMH.v20i1.2642
PREPARATION AND VALIDATION OF A SYSTEMIC LOXOSCELISM PREDICTION PROTOCOL
ELABORACIÓN Y VALIDACIÓN DE UNA REGLA DE PREDICCIÓN CLINICA PARA IDENTIFICAR COMPROMISO SISTEMICO EN CASOS DE LOXOSCELISMO
Rafael Pichardo-Rodriguez1,
Marcos Saavedra-Velasco2,
Jhonatan Ascarza-Saldaña1,
Cesar Naquira-Velarde3
1Advanced Urology Clinic UROZEN, Lima, Peru.
2National Hospital Edgardo Rebagliati Martins, Lima, Peru.
3Research Institute in Biomedicine. Ricardo Palma University, Lima, Peru
ABSTRACT
Introduction: Systemic loxoscelism is the most severe complication of loxoscelism. The management of the cadre by health personnel presents a high variability due to factors that are currently unknown. There is no standard of reference or a clinical prediction model that can guide our decisions when approaching a spider bite patient. Objective: Develop and validate a clinical prediction rule for systemic loxoscelism. Methods: An observational study of derivation and validation of a clinical prediction model was carried out with diagnostic test validation based on a historical single-arm cohort in patients treated at Vitarte Hospital between 2007 and 2016 and international clinical reports published. Results: Systemic loxoscelism occurred only in 32.9% (n = 24) of cases. For the bivariate analysis, the variables that showed a statistically significant association (P <0.05) were sex, bite in an independent abdomen in relation to other parts of the body, bite in other parts of the body than the abdomen, vomiting , fever and hemoglobinuria. The regression analysis included in the analysis the variables: sex, vomit, fever and hemoglobinuria. Bootstrapping determined the internal validity of the model. The area under the curve was 0.91 (P <0.05) and the sensitivity, specificity, LR + and LR- were 79.1%, 93.8%, 12.9 and 0.22 respectively. Conclusions: The protocol of prediction of systemic derived loxoscelism is valid, for the moment.
Keywords: Spider venoms; Brown Recluse Spider; Prediction.
RESUMEN
Introducción: El loxoscelismo sistémico es la complicación más severa del loxoscelismo. El manejo del cuadro por parte del personal de salud presenta una alta variabilidad por factores que se desconocen actualmente. No se cuenta con un estándar de referencia ni con un modelo de predicción clínica que pueda guiar nuestras decisiones al momento del abordaje de un paciente con mordedura de araña. Objetivo: Elaborar y validar una regla de predicción clínica para loxoscelismo sistémico. Métodos: Se llevó a cabo un estudio observacional de derivación y validación de un modelo de predicción clínica con validación de prueba diagnóstica basada en una cohorte histórica de un solo brazo en pacientes atendidos en el Hospital Vitarte entre los años 2007 al 2016 y reportes clínicos internacionales publicados. Resultados: El loxoscelismo sistémico se presentó solo en el 32,9 % (n=24) de casos. Para el análisis bivariado, las variables que demostraron presentar una asociación estadísticamente significativa (P<0,05) fueron el sexo, mordedura en abdomen independiente en relación a otras partes del cuerpo, mordedura en otras partes del cuerpo que no sea el abdomen, vómito, fiebre y hemoglobinuria. El análisis de regresión incluyó en el análisis a las variables: sexo, vómito, fiebre y hemoglobinuria. El bootstrapping determinó la validez interna del modelo. El área bajo la curva fue de 0,91 (P<0,05) y la sensibilidad, espeficidad, LR+ y LR- fueron de 79,1%, 93,8%, 12,9 y 0,22 respectivamente. Conclusión: El protocolo de predicción del loxoscelismo sistémico derivado es válido, por el momento.
Palabras Clave: Venenos de arañas; Araña Reclusa Parda; Predicción.
Diagnosis and prediction of systemic loxoscelism keeps being a mystery and a challenge for the medical doctor who handle patients with spider bite. There is no reference standard for diagnosis, despite researches and development of rapid tests of immunoassays (ELISA). These ELISA rapid tests have demonstrated a successful outcome in European countries, despite they have been developed based on North American Loxosceles species. However, they are not validated for different populations and they are not available in all countries(1–3). Furthermore, we have not been able to establish so far a prediction for the poisoning evolution, which is a key point for managing and reducing mortality, morbidity and costs for the patient and national health budget. Although there are variables or factors associated to systemic loxoscelism, like having a bite to the level of the thorax, fever and bad general condition within the first 24 hours; as Webb et al(4), reports, there is no evaluation of neither your diagnostic nor predictive capacity.
In addition, there is a great variability in diagnosis and treatment between medical doctors, a reality which is observed in both national and international level, because we cannot rely on standardized procedures, nor with a clinical practice guideline. That is why the objective of this study was to elaborate and validate a clinical prediction rule in order to identify cases of systemic compromise in patients treated for loxoscelism.
METHODS
Study design, place and time
We carried out an observational study of derivation and validation of a clinical prediction rule based on a sole-arm cohort in patients treated at Vitarte Hospital between the years 2007 and 2016, and published international clinical reports.
Since it is a low-frequency pathology, we replicate the recruitment method of two patients’ origins applied by Xian Han et al(5) in a prospective study of an extremely rare hematological malignancy. Certain patients’ origins are those who are treated at hospital and others origins are those presented in scientific publications (case reports).
Selection criteria
We included those patients treated at the Vitarte Hospital between the years 2007 and 2016 with confirmed diagnosis of loxoscelism (cutaneous and systemic) and that case report published on international database (web of science, scopus, pubmed y scielo) and who presented study variables (bite on thorax, abdomen, fever onset, nausea, vomiting and bad general condition within the first 24 hours of evolution, jaundice, hemoglobinuria, high levels of total bilirubin, creatinine and LDH) after the bite. We excluded those patients with missing or incomplete medical history and those who presented cutaneous anthrax, spider bite of other genus, insect bites (African bee and/or Lonomia sp), herpes simplex chronic infected, diabetic ulcer, toxic epidermal necrolysis and erythema nodosum multiforme.
Definition of illness, variables and endpoints
We defined systemic loxoscelism as a genus Loxosceles spider bite associated to any organ involvement but the skin (hemolytic anemia, renal failure, acute hepatic failure, etc.). In addition, we studied the following variables: sex, age, bite on thorax, abdomen, fever onset, nausea, vomiting and bad general condition within the first 24 hours of evolution, jaundice, hemoglobinuria, high levels of total bilirubin, creatinine and LDH.
Methods and procedures for variables measurement and collection
Data was registered based on medical records from patients on data sheets elaborated on the basis of the study objectives. Follow-up time: The participants were followed and controlled since the bite, until 5 days after the accident.
a) Data collection from international publications
We placed health sciences descriptors (DeCS) and “Medical Subject Headings” (MeSH): Loxoscelismo, loxoscelism, Loxosceles, Loxosceles Reclusa, Case Reports, reporte de caso, serie de caso, series case, Spider Venom, Brown Recluse Spider. In the different international databases: PUBMED, Web of Science, Ovid PubMed, LILACS, LIPECS, SCIELO and Open Grey. We included those case reports and series where the accident was compatible with the loxoscelism clinic, confirming the diagnosis and/or we identified the spider. We collected data on data sheets based on selection criteria.
b) Data collection from patients treated at Vitarte Hospital:
We sent an acknowledgement and enforcement request of the thesis project to the teaching and research support office from Vitarte Hospital, after the approval of the project by the Faculty Council and Research Institute in Biomedicine (INICIB, for its acronym in Spanish) of the Faculty of Medicine “Manuel Huamán Guerrero” of Ricardo Palma University.
Subsequent to the approval, we requested the Statistical Office for the number of loxoscelism cases concerning the spider venom code CIE-10: T63.3, obtaining the number of patients and their medical record number since the year 2007 until 2016 with spider accident. We placed every medical record and selected those who met selection criteria, collecting necessary information on data sheet.
Calculation of sample size
For a 95% confidence level, with an expected proportion of loxoscelism cases with a 5% systemic compromise and a 5% precision, we required a total of 73 patients in order to complete the study. Sample selection was made for convenience, including every found case.
Ethical aspects
We did not experiment with human beings. We maintain and protect data confidentiality, every participant had right to anonymity, thus, respecting the principles of good clinical practice proposed on the Declaration of Helsinki.
Statistical analysis
We carried out a statistical descriptive analysis with presentation of percentage and frequencies for qualitative variables, and Medias, standard or median deviation and range for quantitative ones, on the basis of the results of the normality tests using Shaphiro-Wilk. Afterwards, we carried out a bivariate analysis evaluating the association between possible predictors (we considered a p<0.05 as statistically significant). For the statistical association among qualitative variables with more than two categories, we performed Bonferroni’s Post Hoc test for Pearson’s Chi square, considering statistically significant a p<0.05 value. Those variables with a “p” value under 0.05 were included in the binary logistic regression model through “STEPwise” method. Regarding variables selected by the regression model of our prediction protocol, there was presence of their respective Odds Ratio (OR) with their respective confidence interval at 95% (CI-95%) and p value. We evaluated discriminative capacity of protocol through analysis of receiver operative curves (ROC). Protocol cut-off points were obtained on basis of likelihood ratio determination (LR), both positive (LR+) and negative (LR-), and Youden’s index. We calculated: sensitivity, specificity for the best cut-off point. In order to determine model’s internal validity, we performed a re-sampling with 50 repetitions through Bootstrapping technique, presenting their respective origin, boot and bias’ coefficient. Each coefficient represented a specific score per each variable, constituting prediction protocol, presenting per each score as well as each symptom or sum of them its respective likelihood of systemic loxoscelism. We developed a Fagan’s nomogram based on LR+ and pre-test and pro-test probabilities from simulating three different clinical scenarios where we applied prediction protocol (Scenario 1 [Red Line] a medical doctor presents a 10% pre-test likelihood in a patient who attends his/her office for a spider bite. Scenario 2 [Green line] same medical doctor presents a 50% pre-test likelihood. Scenario 3 [Blue line] same medical doctor presents 80% pre-test likelihood in a patient who shows another spider bite). Data were processed and analyzed in statistical software STATA Version 14 (Stata Corp. Texas, U.S.).
RESULTS
For the analysis, we included 73 patients, of which 49 came from case reports and series Table 1 and 24 from Vitarte hospital. Of all, systemic loxoscelism was present in 32.9% (n=24) of cases, of which 21 cases came from case reports and series, and 3 cases from Vitarte hospital. Clinical features of patients with loxoscelism can be seen in Table 2.
Variables: summertime accident, accident in other times of the year, nausea, bad general condition, low level of hemoglobin, high level of indirect bilirubin, creatinine, LDH, prolongation of prothrombin and complications, presented lost data in more than 50% making impossible its use.
For the bivariate analysis, variables which demonstrated a statistically significant association with the developing of systemic situation of loxoscelism were age, sex, bites on any part of the body but the thorax, vomiting, fever and hemoglobinuria. Variables: bite on the thorax, pallor, and jaundice presented within contingency table a 0 value, which makes impossible to calculate statistical bivariate analyses, reason why we did not use the results for the following statistical analyses. Bonferroni’s Post Hoc test for Pearson’s Chi square provided a statistically significant result (P=0.02) for bite on the abdomen in comparison with different categories. See results in Table 1.
Regression analysis included variables: sex, vomiting, fever and hemoglobinuria. We did not consider variables: age, bite on thorax nor bite on any part of the body but the abdomen. In Table 2 we sum up the results.
Prediction model showed an area under the 0.91 curve (P< 0.05), with a 0.73 Youden’s index and a 0.35 classification criterion or cut-off point for prediction value calculated through the model, being equivalent to a score of 4 in the derivate prediction rule. Sensitivity was 79.1% and specificity, 93.8%, LR+ was 12.9 and LR-, 0.22. In Figure 1, you can see ROC curve of predictive model.
Bootstrapping to determine model’s internal validity for 50 repetitions showed origin and boot coefficients of 0.68 and 0.69 (CI-95%: 0.68-0.70) and bias was only of 0.008 (CI-95%: 0.007-0.009), corroborating model’s internal validity.
Derivate clinical prediction rule with its respective score it’s shown in Table 3.
Results of derivate protocol scores are:
• 2 points: 14% likely to develop systemic loxoscelism
• 3 points: 22% likely to develop systemic loxoscelism
• 4 points: 57% likely to develop systemic loxoscelism
• 9 points: 98% likely to develop systemic loxoscelism
• 11 points: 99% likely to develop systemic loxoscelism
If we mix chances of symptoms presented by patients, we obtain:
• Male sex: 14% likely to develop systemic loxoscelism
• Fever: 22% likely to develop systemic loxoscelism
• Vomiting: 14% likely to develop systemic loxoscelism
• Hemoglobinuria: 57% likely to develop systemic loxoscelism
• Male sex + fever: 62% likely to develop systemic loxoscelism
• Male sex + fever + hemoglobinuria: 98% likely to develop systemic loxoscelism
• Male sex + fever + vomiting + hemoglobinuria: 99% likely to develop systemic loxoscelism
In Figure 2, we display Fagan’s diagram, which shows three clinical scenarios: Scenario 1 (Red line) a medical doctor presents a 10% pre-test likelihood in a patient who attends his/her office for a spider bite, and when he/she applies the predictive model, the patient presents a 64% post-test likelihood to have systemic loxoscelism. Scenario 2 (Green line) same medical doctor presents a 50% pre-test likelihood in a patient with another spider bite, post-test likelihood rises to 95%. Scenario 3 (Blue line) same medical doctor presents a 80% pre-test likelihood in a patient with another spider bite, and when he/she applies the predictive model, post-test likelihood rises to 99%.
Table 2. Clinical features of patients with loxoscelism.
| Systemic loxoscelism (n=24) | Cutaneous loxoscelism (n=49) | P value | |
| Age | 11 years old (Range 0.3-92) | 28 years old (Range 1-80) | P=0.020 |
| Sex | |||
| Female | 6 (25%) | 30 (61.2%) | P=0.040 |
| Male | 18 (75%) | 19 (38.8%) | |
| Time of sickness | |||
| Less than 24 hours | 9 (37.5%) | 25 (51%) | P=0.270 |
| More than 24 hours | 15 (62.5%) | 24 (49%) | |
| Injury site | |||
| Abdomen | 5 (20.8%) | 2 (4,1%) | P=0.080 |
| Face | 1 (4.2%) | 7 (14.3%) | |
| Neck | 2 (8.3%) | 1 (2%) | |
| Back | 1 (4.2%) | 0 (0%) | |
| Scrotum | 0 (0%) | 1 (2%) | |
| Breast | 0 (0%) | 1 (2%) | |
| Lower limb | 6 (25%) | 19 (28.8%) | |
| Upper limb | 7 (29,2%) | 11 (22.4%) | |
| Ear | 1 (4,2%) | 2 (4.1%) | |
| Eyelid | 0 (0%) | 5 (10.2%) | |
| Thorax | 1 (4.2%) | 0 (%) | |
| Bite on Thorax | |||
| Yes | 1 (4.2%) | 0 (0%) | Uncalculable* |
| No | 23 (95.8%) | 0 (0%) | |
| Bite on Abdomen** | |||
| Yes | 5 (20.8%) | 2 (4.1%) | P=0.020 |
| No | 19 (79.2%) | 47 (95.9%) | |
| Bites on any part of the body but the abdomen | |||
| Yes | 18 (75%) | 47 (95.9%) | P<0.007 |
| No | 6 (25%) | 2 (4.1%) | |
| Vomiting | |||
| Yes | 12 (50%) | 2 (4.1%) | P=0.001 |
| No | 12 (50%) | 47 (95.9%) | |
| Fever | |||
| Yes | 16 (66.7%) | 6 (12.2%) | P=0.001 |
| No | 8 (33.3%) | 43 (87.8%) | |
| Pallor | |||
| Yes | 15 (62.5%) | 0 (0%) | Uncalculable |
| No | 9 (37.5%) | 49 (100%) | |
| Jaundice | |||
| Yes | 10 (41.7%) | 0 (0%) | Uncalculable |
| No | 14 (58.3%) | 49 (100%) | |
| Hemoglobinuria | |||
| Yes | 12 (50%) | 1 (2%) | P=0.001 |
| No | 12 (50%) | 48 (98%) | |
| Death | |||
| Yes | 4 (15%) | 2 (4.1%) | P=0.087 |
| No | 20 (75%) | 47 (95.9%) | |
** Statistically significan result of Bonferroni’s Post Hoc test for Pearson’s Chi square.
Table 1. Case reports and series included in the study.
| Type of article | Autor et al | Systemic Loxoscelism | Age | Sex | Injury site | Time of sickness | Death |
| Case Report | No | 5 | Male | Abdomen | More than 24 hours | No | |
| Case Report | No | 28 | Male | Upper limb | More than 24 hours | No | |
| Case Report | No | 7 | Female | Eyelid | Less than 24 hours | No | |
| Case Report | No | 10 | Female | Lower limb | More than 24 hours | No | |
| Case Report | No | 7 | Male | Eyelid | More than 24 hours | No | |
| Case Report | No | 14 | Female | Abdomen | Less than 24 hours | No | |
| Case Report | No | 15 | Male | Upper limb | Less than 24 hours | No | |
| Case Report | No | 15 | Female | Face | Less than 24 hours | No | |
| Case Report | No | 14 | Male | Neck | Less than 24 hours | No | |
| Case Report | No | 80 | Male | Upper limb | Less than 24 hours | No | |
| Case Report | No | 7 | Male | Lower limb | More than 24 hours | No | |
| Case Report | No | 10 | Female | Upper limb | More than 24 hours | No | |
| Case Report | No | 34 | Female | Eyelid | More than 24 hours | No | |
| Case Report | No | 7 | Female | Eyelid | More than 24 hours | No | |
| Case Report | Tarullo et al | No | 31 | Male | Lower limb | More than 24 hours | No |
| Case Report | Zagouri et al | No | 56 | Male | Breast | Less than 24 hours | No |
| Case Report | Christopher et al | No | 48 | Female | Lower limb | Less than 24 hours | No |
| Case Report | Entrambasaguas et al | No | 27 | Female | Face | More than 24 hours | No |
| Case Report | Raymond et al | No | 45 | Female | Lower limb | More than 24 hours | No |
| Case Report | Rojas et al | No | 62 | Female | Lower limb | More than 24 hours | No |
| Case Series | Sams et al | No | 31 | Female | Lower limb | More than 24 hours | No |
| Case Report | Stoecker et al | No | 10 | Female | Upper limb | Menos de 24 horas | No |
| Case Report | Aguilar et al | No | 25 | Male | Lower limb | Menos de 24 horas | No |
| Case Report | Robert M. Jarvis | No | 8 | Female | Face | Menos de 24 horas | No |
| Case Report | A.Z. Bhatti | No | 38 | Male | Lower limb | Menos de 24 horas | No |
| Case Report | I. Holtslag | No | 22 | Female | Ear | Menos de 24 horas | No |
| Case Report | D. RIBUFFO | No | 20 | Female | Eyelid | Menos de 24 horas | No |
| Case Report | Yes | 0.3 | Male | Upper limb | Less than 24 hours | Yes | |
| Case Report | Zambrano | Yes | 71 | Male | Back | More than 24 hours | Yes |
| Case Report | Yes | 0.9 | Female | Lower limb | More than 24 hours | No | |
| Case Report | Yes | 10 | Male | Neck | Less than 24 hours | No | |
| Case Report | Stoecker et al | Yes | 16 | Male | Face | More than 24 hours | No |
| Case Report | Cabrerizo et al | Yes | 6 | Male | Abdomen | More than 24 hours | No |
| Case Report | Said et al | Yes | 6 | Male | Abdomen | Less than 24 hours | No |
| Case Report | Carina Levin et al | Yes | 3 | Female | Lower limb | Less than 24 hours | No |
| Case Report | Vichal et al | Yes | 50 | Male | Upper limb | More than 24 hours | No |
| Case Report | Wilson et al | Yes | 24 | Female | Lower limb | More than 24 hours | No |
| Case Report | Alfaro et al | Yes | 92 | Male | Upper limb | More than 24 hours | No |
| Case Report | Luna Garcia | Yes | 19 | Male | Lower limb | More than 24 hours | No |
| Case Report | Tolwani et al | Yes | 21 | Male | Abdomen | More than 24 hours | No |
| Case Report | Lane et al | Yes | 19 | Female | Lower limb | More than 24 hours | Yes |
| Case Report | Lane et al | Yes | 9 | Female | Abdomen | Less than 24 hours | No |
| Case Report | Naj et al | Yes | 23 | Male | Upper limb | More than 24 hours | No |
| Case Report | Carina Levin | Yes | 3 | Male | Lower limb | Less than 24 hours | No |
| Case Report | Leanna Lane | Yes | 9 | Male | Neck | Less than 24 hours | No |
| Case Report | Sreekrishna Kanth Donepudi | Yes | 11 | Male | Ear | More than 24 hours | No |
| Case Report | Karen M. Rogers | Yes | 11 | Male | Upper limb | More than 24 hours | No |
| Case Report | JesYesca L. Rosen | Yes | 3 | Male | Thorax | Less than 24 hours | Yes |
| Case Report | M.E. MICHAUD | Yes | 6 | Male | Upper limb | Less than 24 hours | No |
Tabla 3. Results of logistic regression analysis.
| Coefficient | OR | CI-95% | P value | |
| Sex | 1.8 | 6.6 | 1.1-40.2 | 0.039 |
| Vomiting | 2.3 | 10 | 1.8-55.6 | 0.008 |
| Fever | 3.1 | 21.7 | 2.2-215.7 | 0.008 |
| Hemoglobinuria | 3.9 | 52.6 | 2.9-928.1 | 0.006 |
Table 4. Systemic loxoscelism prediction protocol.
| Score | |
| Male sex | 2 |
| Vomiting | 2 |
| Fever | 3 |
| Hemoglobinuria | 4 |
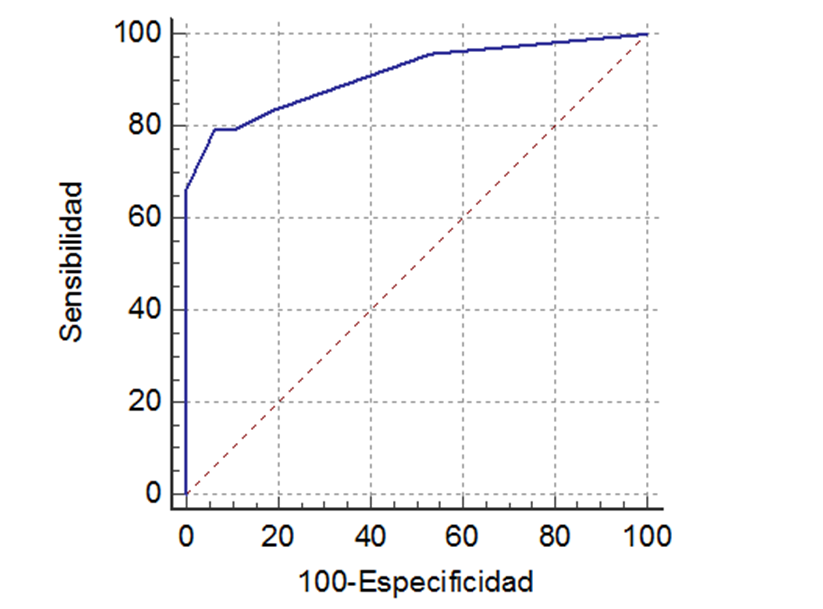
Figure 1. ROC curve of clinical prediction protocol of systemic loxoscelism. Source: from same author.
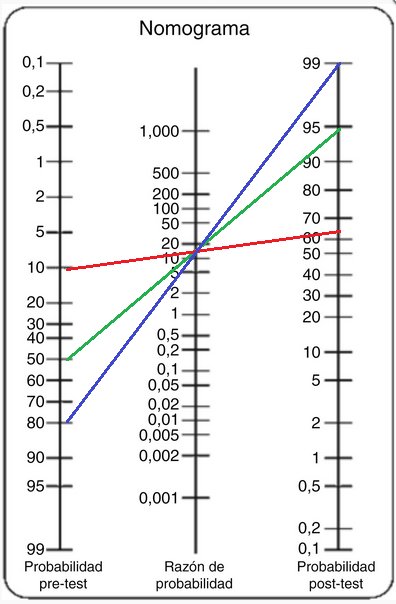
Figure 2. Fagan’s nomogram, which shows three different clinical simulated scenarios (Blue, red and Green line). Source: from same author.
According to the objective of the study, we could develop and validate a prediction protocol for systemic loxoscelism. Although clinical predictive rules are a useful tool to guide decision-making in two specific clinical scenarios, as diagnosis and prognosis of diseases and other clinical endings are, they are only a guide but are not a determinant in decision-making(6). Prediction protocol of derivate loxoscelism is the first of its kind and we expect that it may help guiding clinical decisions, in both improving case detection and saving of health resources. The fact that draws the attention is that variables included in the prediction model (vomiting, fever, hemoglobinuria) are variables which have been studied and have demonstrated statistical association in previous research, as it is reported in studies by Webb et al and others(4,7-9); however, we also included another variables like male sex, which does not present any research regarding its association with systemic loxoscelism. Furthermore, Dahod et al(10), determined predictors of renal failure in a snake bite. They found that hypotension, albuminuria, bleeding time, prothrombin time, hemoglobin, total bilirubin and time from bite until hospital admission, properly predicted development of acute renal failure. However, in our study, we could not include these variables in statistical analysis due to great quantity of lost data, which may be the result of great variability of medical management by medical personnel. It is important to consider in further studies the evaluation of these variables in terms of systemic loxoscelism, because the clinical picture produced by snake bite with vascular-toxic effect is similar to the clinical picture originated by Loxosceles, which also includes acute renal failure.
Median age of the group which presented systemic loxoscelism was statistically different to the median age of the group which did not have it. Webb et al(4), reports that age range of patients with systemic loxoscelism is 21 to 61 years, and that there are not significant differences between both groups. Moreover, Malaque et al(9), also report that there are no differences between patients’ age ranges with systemic and cutaneous loxoscelism. In addition, in another study, they show that the most frequent age range is 30 to 44 years. Age plays an important role at the time of diagnosis. Since patients that are in extreme ages groups (2 years old or 90 years old) are the most likely to develop systemic loxoscelism, this variable in our study demonstrated that it is a significantly associated variable, which has not evidence any association in other studies. However, on basis of variability among case reports and series, more studies should be carried out to evaluate its behavior in further studies.
The most affected gender by systemic loxoscelism was male. Although loxoscelism is known for affecting more frequently feminine gender, as it is reported in a paper by the Epidemiology Department regarding loxoscelism situation in Peru year 2013(11), as well as the frequency reported by Nuñez et al(12) in a study carried out at a Social Security EsSalud Hospital, and the frequency by Valverde(13), in a study accomplished at the Docente de Trujillo Regional Hospital. Webb et al(4) find that loxoscelism affected more frequently men in comparison with women. Malaque et al(9), report that masculine gender prevailed in systemic loxoscelism cases, where indirect bilirubin was greater than 7. We recommend that, at the time of clinical approach, a variable to be considered for diagnosis and management of a patient suspected of having systemic loxoscelism should be male sex. Even though, in our study, we did not included it in the prediction model by regression analysis, it is a clinically important variable.
Time of sickness greater than 24 hours was the most frequent in the systemic loxoscelism group. It is known to be systemic loxoscelism if a patient with spider bite presents himself/herself within the first 24 hours, being more likely to be cutaneous loxoscelism after this period of time(14). A current review by Maguiña et al(15), mentions that time for systemic loxoscelism development could reach 36 hours after the accident. Our results affirm that it is likely to appear after this period of time, hence, we can conclude that, regardless of evolution time, diagnosis should be based on other patient’s features that joined together, they should give us an approximate likelihood of the case. Our prediction protocol could resolve this gap in the Peruvian level.
At the moment of the accident, different parts of the body seem affected, more frequently the limbs(14). Nuñez et al(12), reports that the most frequent place where loxoscelism shows up are lower limbs, being similar to the results found in our study population. Furthermore, there were body areas, like bite to the level of the Abdomen, which were significantly associated to systemic loxoscelism’s presentation, agreeing with Webb et al(4) report, who find a bite on Abdomen is statistically associated to development of systemic loxoscelism. Even though this variable shows association, it was not included in the prediction model, hence, it needs an evaluation in terms of a bigger population in the future. However, it may guide our clinical decisions. Bite in any part of the body but the Abdomen was statistically associated to a lower likelihood of developing systemic loxoscelism. Although, physiopathologic cause is unknown, it is possible that this association may be due to anatomical and physiological features of the different body areas. It is clinically important to consider, that every patient with bite to the level of the thorax, is likely to develop systemic loxoscelism.
When evaluating statistical association and predictability of the different studied variables, the one which showed a greater importance by its association strength and high capacity to predict systemic loxoscelism was hemoglobinuria. It is known that hemoglobinuria is the result of hemolysis and saturation of haptoglobin, which leads to elevated excretion of hemoglobin by kidneys, clinically seen as dark urine or hemoglobinuria, causing renal damage due to a renal tamponade; and that inflammatory changes that it produces in renal tubules can make the patient evolve towards an acute tubular necrosis in many cases(16). Although, it is known that acute renal failure, main complication and cause of death of systemic loxoscelism, is caused by renal tamponade, it has been demonstrated that venom also presents direct impact in terms of renal tubules’ cells, and that immune system plays a fundamental role inside the pathology(16,17). Sezerino et al(18), state that only 28.6% of patients with systemic loxoscelism presents hemoglobinuria. Webb et al(4), report a different number, reaching to 48 the percentage of patients with systemic loxoscelism who presents hemoglobinuria. Alva-Medina et al(19) report a 61.1% hemoglobinuria frequency in kids with systemic loxoscelism in Peru. When comparing these evidences with our results, in fact, it turns out that hemoglobinuria is an important variable to consider when diagnosing systemic loxoscelism, demonstrating its high predictive power in our findings. However, it may not be present in every case. That is the reason why the clinical prediction model must be validated in a bigger population.
Fever is an associated symptom to systemic loxoscelism and it could alert us about the presence of systemic loxoscelism in a patient with spider bite(15). Fever is also associated with presence of hemolysis(9). However, Malaque et al(20), report that only 16% of patients with loxoscelism has a low grade fever. In another study carried out by the same author and partners, they find the opposite result, that fever is present in 68% of patients suspected to have systemic loxoscelism(9). Fever’s predictability has shown an important role in our protocol, being important its consideration when addressing to a patient suspected to have systemic loxoscelism. Vomiting is another important variable to consider in diagnosis, and it is outlined as a symptom which may be observed in systemic loxoscelism(15,14). This variable, along with the previous ones, is clinically relevant for diagnosis. As a result, we obtain an improvement in the management of systemic loxoscelism and a reduction in health resources expenses. We can also predict when a patients can or cannot develop a systemic loxoscelism after a bite, which would be added value of our prediction model.
Mortality in both groups was low. In cutaneous loxoscelism group, the patient passed away for an infection associated to skin conditions. In this aspect, clinical management of systemic loxoscelism becomes important, which is mainly supporting(15). A proper management determines good or bad outcome of patients, and it influences directly in terms of mortality. In that regard, management of these cases was proper apparently, thus, mortality was low regarding systemic loxoscelism.
First limitation is the reduced number of sample size, which affects directly statistical power and generalization of results for bigger populations. However, diversity of included cases belonging to different parts of the world makes the model also applicable for populations, in both inside and outside Peru, turning the applied methodology in a contribution for studying rare disease. Second limitation is the important quantity of lost data, which conditioned deleting a significant part of operationalized variables for statistical analysis. However, they were not determinant neither in the development of this research nor in the derivation of the prediction model. Nonetheless, they limited including other variables. That is the reason why recommend replicating this study in a bigger and multicenter population, in order to guarantee sample power, robust findings, inclusion of other variables and greater generalization of results, and benefit those populations which does not share the same features of the studied population.
Clinical prediction rule of derivate systemic loxoscelism is valid and applicable to populations which are inside and outside Peru. Applied methodology is a contribution for studying rare disease. An external validation in a bigger population is required: evaluation in real clinical conditions and inclusion of additional variables.
Acknowledgements: To Dr. Alonso Soto-Tarazona, for
the support provided in the conception of the idea and
the study design.
Authorship Contributions: The authors participated
in the genesis of the idea, project design, data collection
and interpretation, analysis of results and preparation
of the manuscript of this research paper.
Financing: Self-financed.
Interest conflict: The authors declare no conflict of
interest in the publication of this article.
Received: November 07, 2019
Approved: December 30, 2019
Correspondence: Rafael Pichardo-Rodriguez
Address: Dirección: Av. Javier Prado Este 3028, San Borja 15037
Telephone: 986332210
E-mail: rafael_martin1352@hotmail.com
BIBLIOGRAPHIC REFERENCES
