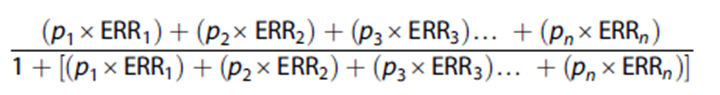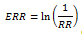ORIGINAL ARTICLE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2019 - Universidad Ricardo PalmaDOI 10.25176/RFMH.v20i1.2657
FEASIBILITY STUDY TO EVALUATE THE PROPORTION OF CANCER ATTRIBUTABLE TO MODIFICABLE RISK FACTORS IN PERU AND LATIN AMERICA .
ESTUDIO DE FACTIBILIDAD PARA EVALUAR LA PROPORCION DE CANCER ATRIBUIBLE A FACTORES DE RIESGO MODIFICABLES EN EL PERÚ Y LATINOAMERICA.
Jhony A. De La Cruz-Vargas1,4
, Willy Ramos2,3
, Willer Chanduví1
, Rubén Espinoza1
, Nadia Guerrero2
, Joan A. Loayza-Castro1
, Alfonso Gutiérrez Aguado1,2
, Ronald Carpio1
, Manuel Loayza Alarico1,2
1 Research Institute in Biomedical Sciences (INICIB), Ricardo Palma University, Lima-Peru.
2 National Center for Epidemiology, Disease Prevention and Control, Ministry of Health, Lima-Peru.
3 Institute of Clinical Research, National University of San Marcos, Lima-Peru.
4 Latin American Lifestyle Medicine Association.
ABSTRACT
Objective: To evaluate the feasibility and validate the methodological proposal to estimate the incidence and mortality due to cancer attributable to modifiable risk factors for Peru and Latin America.
Methods: Pilot study, ecological from secondary sources. Modifiable risk factors, exposure prevalence, relative risks of these factors (RR) or an approximation by means of possibilities ratio (OR) were searched and selected. The information was recorded in a data collection form which was validated by expert judgment. For the calculation of the Population Attributable Fraction (FAP), the formula proposed by Parkin was tested and a statistical simulation model was developed with R. Studio V. 3.6.1 software.
Results: In Peru there are prevalence studies for the majority of modifiable risk factors; Likewise, studies with OR estimates for several of the factors are available in Latin America; however, studies from the United States had to be used for the remaining factors. No national studies of ionizing or ultraviolet radiation were found. The syntax of the statistical simulation model was tested, which proved to be valid and consistent with the results of international FAP studies within the ranges of published studies.
Conclusion: It is feasible and viable to carry out PAF studies of modifiable risk factors for cancer in Latin American countries, particularly in Peru, where the information required for its estimation is available.
Key words: Cancer; Risk factors; Population attributable fraction; Lifestyle medicine (source: MeSH NLM).
RESUMEN
Objetivo: Evaluar la factibilidad y validar la propuesta metodológica para estimar la incidencia y mortalidad por cáncer atribuible a factores de riesgo modificables para el Perú y Latinoamérica.
Métodos: Estudio piloto, ecológico a partir de fuentes secundarias. Se buscó y seleccionó los factores de riesgo modificables, prevalencia de exposicion, los riesgos relativos de dichos factores (RR) o una aproximación mediante la razón de posibilidades (OR). La información fue consignada en una ficha de recolección de datos la cual fue validada mediante juicio de expertos. Para el cálculo de la Fracción Atribuible Poblacional (FAP) se ensayó la fórmula planteada por Parkin y se desarrolló un modelo de simulación estadística con el software R. Studio V. 3.6.1.
Resultados: En el Perú se cuenta con estudios de prevalencia para la mayoría de factores de riesgo modificables; asimismo, se dispone en Latinoamérica de estudios con estimaciones de OR para varios de los factores; sin embargo hubo que utilizar estudios de los Estados Unidos para los factores restantes. No hallamos estudios nacionales de radiaciones ionizantes ni ultravioleta. Se ensayó la sintaxis del modelo de simulación estadística la cual mostró ser válida y consistente con los resultados de investigaciones internacionales de FAP encontrándose dentro de los rangos de los estudios publicados.
Conclusión: Es factible y viable realizar estudios de FAP de factores de riesgo modificables para cáncer en países de Latinoamérica, particularmente en el Perú, donde se cuenta con la información requerida para su estimación.
Palabras Clave: Cáncer; Factores de riesgo, Fracción atribuible poblacional; Medicina del estilo de vida (fuente: DeCS BIREME).
Cancer is one of the main causes of morbidity and mortality and is greatly influenced by lifestyle and environmental risk factors. The risk factors that contribute to the general burden of cancer are those with the highest relative risks associated with exposure, with the highest prevalence of exposure in the population and with the highest number of associated common types of cancer or combinations thereof. .. The prevalence of exposure to these risk factors varies with the sex, time, geography and ages eligible for screening programs.(1-3)
Because of this, many cancers are causally related to potentially modifiable risk factors and estimates of this proportion in a population are known as the population attributable fraction (PAF). Thus, PAF is a valuable tool for establishing priorities for cancer prevention and control. (4,5).
Changes in exposure to modifiable risk factors are key drivers of changes in cancer incidence and mortality and improvements in the diagnosis of cancer. The quantification of the participation of these risk factors would contribute to the reduction in the incidence of cancer, which could be achieved by reducing or eliminating risk exposure. Therefore, primary prevention through modifications of these lifestyle and environmental factors at the population level, now known as Lifestyle Medicine(6), offers great possibilities to reduce the number of people diagnosed with cancer. However, estimates of cancer cases attributable to modifiable risk factors are lacking in Peru.
The objective of this study was to evaluate the feasibility and validate the methodological proposal for the estimation of cancer incidence and mortality attributable to potentially modifiable risk factors in Peru during 2018, using nationally representative data in adults over 30 years for 20 types of cancer (excluding nonmelanoma skin cancer).
METHODS
Pilot, quantitative, ecological study conducted from secondary sources.
The modifiable risk for cancer was necessary to previously obtain information on the prevalence of exposure and relative risk according to the sex of these factors from secondary sources. For this, a systematic search of the modifiable risk factors was carried out with a sufficient level of evidence available to be included.
Prevalence of Exposure, Incidence, and Mortality from cancer
The prevalence of exposure to modifiable risk factors for cancer was obtained from the following sources of information.
- World Cancer Observatory (GLOBOCAN-Cancer today): From the estimates of the International Agency for Research on Cancer (IARC) the number of incident cases of cancer could be obtained. The estimate of the number of cancer cases performed by the IARC is also available for the other Latin American countries(7).
- Vital statistics: The number of deaths can be obtained from the Death Records of each country. In the case of Peru, the information was obtained from the technical document “Analysis of the Situation of Cancer in Peru, 2018” that records information from the Death Registry of the Ministry of Health(8). In the case of a considerable underreporting of mortality, it is possible to approximate the number of deaths using estimation and correction methodologies for underreporting(9). In the case of Latin American countries, information can be obtained from the situational analyzes of cancer(10,11) or mortality atlas published in some countries(12).
- National demographic and health surveys: Most Latin American countries have national surveys that allow the prevalence of modifiable risk factors for cancer and other non-communicable diseases(13-17). In the case of Peru, the required information was obtained from the Demographic and Family Health Survey(13) (ENDES) by the National Institute of Statistics and Informatics (INEI) that collects consumption data cigarettes, alcohol, fruits, and vegetable salad, overweight and obesity. Some food consumption data were obtained from the National Household Survey (ENAHO), also in charge of INEI(18).
- Research articles: Not all information about modifiable risk factors can be obtained from demographic and health surveys, so it was necessary to resort to independent research, ideally of national inference, to obtain information such as the prevalence of oncogenic infections, passive exposure to tobacco smoke, physical activity among others(19-21).
- Thesis repositories: In some cases, it was possible to obtain specific information on pre and postgraduate thesis(22), mainly for rare risk factors or for which few studies are allowing the prevalence or incidence to be approximated.
- Global disease burden study: Information from the global disease burden study by the Institute for Health Metrics and Evaluation (IHME), which at the time of the preparation of this article, had information from 1990 to 2017 may also be useful.
Relative Risk (RR)
To identify the relative risks of each modifiable risk factor, a systematic search (PICOT Question) of articles in Pubmed, Scopus, EMBASE, COCHRANE, SCIELO, and IARC technical documents was necessary. They were considered in the first instance meta-analysis, in case of not finding it was considered cohort studies or case-control studies (in that order of importance)(19-21).
The available literature was reviewed taking into account that in the case of some factors it was necessary to differentiate the RRs according to sex and age groups. If there are differences in risk according to geographical regions, the articles of the country where the study is conducted or those that consider estimates of Latin American countries were preferred. Likewise, studies of risk factors that control the confounding effect of other variables were selected in the first instance from a multivariate statistical analysis. In the case of certain risk factors for which there is little information on the risk of exposure (such as air pollution) and / or for those that affect a small fraction of the population (ionizing radiation, exposure to tanning beds, etc. ) its inclusion was assessed(19-21)
For the factors in which the systematic search did not identify studies with RR for the modifiable risk factors, research was used that obtained the odds ratio (odds ratio) as a statistical approximation of the RR.
Estimation of Population Attributable Fraction
The formula proposed by Parkin et al.(23) was used to calculate the Population Attributable Fraction (PAF).
Where p1 is the proportion of the population at exposure level 1 (and so on) and ERR1 is the relative excess risk (relative risk - 1) at exposure level 1 (and so on). FAP was calculated for the absence or decrease of risk factors; For this, the ERRs were calculated as the natural logarithm of the reciprocal of the relative risk, that is:
To validate the values obtained for the FAPs, a Montecarlo statistical simulation model was used using the R Studio V. 3.6.1 software, to allow the calculation of the PAFs and their respective confidence intervals. This software is free to use and can be run on Windows, Linux, and MacOS. The syntax of the S programming language that was developed by Bell Laboratories was adopted. For the simulation, the distribution of prevalences and risks was taken into account, generating 1000 values resembling real values. In the syntax for the calculation of the FAP and their confidence intervals, functions were used where the risk values and the prevalences were those that changed according to the FAP to be calculated.(20)
The number of cancer cases and deaths attributable to each risk factor according to sex was calculated by multiplying the number of cancer cases or deaths in each sex and age group by the FAP in that age group and sex by adding the results of each group of age.(20)
Instrument
A data collection sheet was designed to record the prevalence of exposure to risk factors for cancer and RR obtained from secondary sources. This instrument was subject to content validity by expert judgment.
RESULTS
Modifiable Risk Factors
The systematic search identified as preventable risk factors based on an increase significant risk of cancer (Table 1) to the following:(24-32)
- Unhealthy lifestyles: Cigarette consumption, passive exposure to tobacco smoke, alcohol consumption, consumption of red and processed meats, low consumption of fruits and vegetables, dietary fiber, physical inactivity, and excess body weight.
- Environmental factors: exposure to ultraviolet (UV) radiation, exposure to ionizing radiation.
- Oncogenic infections: Helicobacter pylori infection, hepatitis B virus (HBV), hepatitis C virus (HCV), human herpesvirus type 8 (VHH-8), human immunodeficiency virus (HIV), lymphotropic virus human T-cell type 1 (HTLV-1) and human papillomavirus (HPV).
Exposure Prevalence
After the systematic review, it was found that it is possible to obtain information regarding modifiable risk factors for cancer in Peru. The main source of information is the population surveys conducted by the INEI that allow capturing prevalence information and confidence intervals according to age groups and sex with a national inference level. ENDES accurately collects risk factors linked to unhealthy lifestyles such as tobacco consumption, alcohol consumption, low consumption of fruits and vegetables, overweight and obesity. The ENAHO, for their part, collect data on food consumption by Peruvian households that would allow them to approximate the consumption of processed meats and fiber. This is shown in table 2.
In the case of oncogenic infections, the main source of information was the articles published in indexed journals that were relevant for obtaining data on the prevalence of HBV, HCV, HTLV-1, and VHH-8. While it is true that these articles presented survey information At a sub-national level or in blood banks, their results are consistent and coincide with the results of surveys from previous years or with those reported in the international literature, so they also constitute an alternative to approximate the prevalence of such infections.
In the case of Helicobacter pylory infection, most studies were conducted in the 1990s and only one recent study was documented in two districts of Lima; however, it also allowed a current approximation of the prevalence of infection by said bacteria. Estimates made by international agencies from various studies under meta-analysis models were also useful to determine the prevalence of some oncopathogens, particularly HPV (ICO) and HIV (UNAIDS). The prevalence of oncogenic infections in Peru is shown in table 3.
No studies were found on the prevalence of exposure to ionizing radiation and exposure to ultraviolet radiation that are representative of the general population of Peru and Latin America, so it would not be feasible to be included in FAP studies.
Relative risks or odds ratios
The systematic search allowed to obtain several studies that reported the strength of association of risk factors according to the type of cancer from meta-analysis; however, almost everyone reported the OR instead of the RR. The punctual estimation of the OR and 95% confidence intervals were obtained from studies in Colombia, Cuba, the United State, and Peru (ver table 4).
Finally, the syntax of the statistical simulation model was tested to estimate the FAP and confidence intervals using the R Studio software based on the prevalence of exposure and approximation to the RR obtained. The model proved to be valid and consistent with the results of international FAP research, within the ranges of published studies.
Table 1. Modifiable risk factors according to type of cancer.
| Risk Factor | Type off Cancer (ICD10) |
| Unhealthy lifestyles | |
| Smoking(33) | Oral cavity oral, pharynx (C00-C14); esophagus; stomach (C15); colorectal (C18-C20, C26.0); liver (C22.0, C22.2-C22.4, C22.7,C22.9); pancreas (C25); nasal cavity / sinuses (C30-C31); larynx (C32); lung, trachea bronchus (C33-C34); cervix(C53);kidney, renal pelvis and ureter( C64-C66); bladder (C67); acute myeloid leukemia C92.0; C92.4-C92.5, C94.0, C94.2). |
| Exposure to cigarette smoke(33) | Lung, bronchial, trachea (C33-C34; only among non-smokers and former smokers) |
| Excess of body weight(26,33) | Esophagus (C15; adenocarcinoma only); stomach (C16; only cardio); colorectal (C18-C20, C26.0); liver (C22.0, C22.2-C22.4. C22.7), C27.9); gallbladder (C23); pancreas (C25); breast (C50; postmenopaúsal cáncer only); cervix (C54-C55); ovary (C56); kidney, renal pelvis (C64-C65); thyroid (C73); multiple myeloma (C90.0,C90.2) |
| Alcohol Consumption(26,27,33), | Lip, oral cavity, pharynx (C00-C14); esophagus (C15; only squamous cell carcinoma); colorectal (C18-C20,C26.0); Liver (C22.0, C22.2-C22.4, C22.7, C22.9) larynx (C32); Breast (C90) |
| Poor feeding(26,34) | Breast (C50), colorectal (C128-C20, C26.0) |
| Physical inactivity(24,26,34) | Colon excluding rectum (C18, C26.9) breast (C50; only premenopausal cancers inversely associated with vigorous activity, postmenopausal cancers inversely associated with All kinds of physical activity); uterine body (C54-C55) |
| Ultraviolet radiation(35) | Melanoma of the skin (C43) |
| Infections | |
| Helicobacter pylori(28,36) | Stomach (C16.1-C16.6) |
| Hepatitis B Virus(31,36) | Hígado (C22.0, C22.2-C22.4, C22.7, C22.9) |
| Hepatitis C Virus(32,36) | Liver (C22.0, C22.2-C22.4, C22.7, C22.9); non Hodgkin lymphoma (C82-C85. C96.3) |
| Herpes type 8 virus(36) | Kaposi's Sarcoma (C46) |
| Human Inmunodeficiency Virus(32,36) | Anus (C21); Kaposi´s sarcoma (C46); Cervix (C53); lymphoma of Hodgkin (C81); non– Hodgkin lymphoma (82-C85, C96.3) |
| Human Papilloma virus(29,36) | Oral cavity (C02-C06); oropharynx, tongue (C01, C09-C10), anus (C21), cervix (C53), vulva (C51), vagina (C52), penis (C60) |
Tabla 2. Secondary sources for obtaining the prevalence of risk factors for cancer related to unhealthy lifestyle in Peru.
| Risk Factor | Prevalence (%) | IC 95% | Source | Reference Population | Link |
| Current tobacco Consumption | 11,2 | 10,6-11,8 | INEI – ENDES 2018 | National (15 or more years) | http://iinei.inei.gob.pe/microdatos/ |
| Tobacco use in the last year | 18,9 | 18,2-19,6 | INEI – ENDES 2018 | Nacional (15 or more years) | http://iinei.inei.gob.pe/microdatos/ |
| Exposure to tobacco from second hand at home | 13,6 | 11,3-16,3 | GYTS PERU; 2014 | National (13 to 15 years old) | |
| Second–hand tobacco Exposure in closed public places | 30,8 | 28,2-33,6 | GYTS PERU 2014 | National (13 to 15 years old) | |
| Overweight | 37,3 | 36,5-38,2 | INEI – ENDES 2018 | National (15 or more years) | http://iinei.inei.gob.pe/microdatos/ |
| Obesity | 22,7 | 22,0-23,5 | INEI – ENDES 2018 | National (15 or more years) | http://iinei.inei.gob.pe/microdatos/ |
| Alcohol consumption in the last year | 68,9 | 68,2-69,7 | INEI – ENDES 2018 | National (15 or more years) | http://iinei.inei.gob.pe/microdatos/ |
| Current alcohol Consumption | 34,5 | 33,6-35,3 | INEI – ENDES 2018 | National (15 or more years) | http://iinei.inei.gob.pe/microdatos/ |
| Red Meat Consumption | 29,0 | MARKET STUDY GFK 2015 | National (18 years old and over) | https://elcomercio.pe/peru/peruanos-consumen-carnes-rojas-anuncio-oms-248193 | |
| Red Meat Consumption | 51,8 | 51,0 – 52,7 | INEI – ENAHO 2018 | (homes last 15 days) | http://iinei.inei.gob.pe/microdatos/ |
| Meat Consumption per capita | 6,20 kg (person/year) | MINAGRI – 2017 | National (18 or more years) | https://peru21.pe/economia/ministerio-agricultura-buscara-aumentar-consumo-carne-76411 | |
| Consumption of processed meats |
Lima: 59% Iquitos: 35% Puno: 29% Cerro de Pasco: 17% |
UPCH thesis | Subnational (18 to 65 years) | Figure 1ª: Pattern of Consumption of Eggs, Meats, Fish, Vegetables and Fruits in Urban Population Samples Of Sea Level and Height of Peru. 2014-16. | |
| Consumption of processed meats | 27,4 | 26,5 – 28.4 | INEI – ENAHO 2018 | National(homes last 15 days) | http://iinei.inei.gob.pe/microdatos/ |
| Low consumption of fruits and vegetables | 89,0 | 88,4-89,5 | INEI – ENDES 2018 | National (15 or more years) | http://iinei.inei.gob.pe/microdatos/ |
| Sedentarism (insufficient physical activity) | 75,8 | 74,2-77,3 | Research Article | National (15 to 69 years) | Tarqui C. Nutr. Clín. Diet. Hosp. 2017; 37(4):108-115 |
| Fiber Consumption | 5,7 | 5,6 – 5,7 | INEI – ENAHO 2018 | National (homes last 15 days) | http://iinei.inei.gob.pe/microdatos/ |
| Ionizing Radiation | No data | No data | No data source | --- | --- |
| Ultraviolet Radiation6 | No data | No data | No data source | --- | --- |
Table 3. Secondary sources for obtaining the prevalence of oncogenic infections in Peru.
| Risk Factor | Prevalence(%) | CI 95% | Reference Population | Source |
| Helicobacter pylori Infection | 63,6 | No confidence intgervals reported | Volunteers from 2 districts of Lima |
Pareja Cruz y col. Horiz Med 2017;17(2): 55-8 |
| HBV Infection | 5,0 | 4,1 - 5,9 | Subnational (18-29 years) | Bernabé Ortiz y col. PLoS One. 2011;6(9):e24721 |
| HCV Infection | 0,8-1,2 | Studies in the general population in the blood banks | Rev. Gastroenterol. Perú; 2009; 29-4: 347-54 | |
| VHH-8 Infection |
Argentina: 4,0 Brasil 2,8 Chile 2,6 Total 3,7 |
3,5 - 6,4 1,0 - 4,6 31,1 - 4,9 2,9 - 4,5 |
Blood donors | J Med Virol. 2004;72(4):661-7. |
| VHH–8 Infection | Peru 6,6 | Blood donors | J Med Virol. 2017 Mar;89(3):518-527. | |
| VIH Infection | 0,3 | 0,3-0,5 | National | ONUSIDA |
| ITLV-1 Infection | 3,4 | 3-4 | Adult blood Donors of the Guillermo Díaz de la Vega Regional Hospital(Abancay). | Ramírez-Soto.Transfus Med. 2018;28(3):263-5. Sánchez- Palacios. Int J Infect Dis 2003;7:132-7. |
Tabla 4. Estimación puntual del OR e intervalos de confianza para factores de riesgo modificables según país y región.
| Exposure Factor / type of cancer | Region | OR | Confidence Intervals (ic: 95%) |
| Physical inactivity | |||
| Ovarian Cancer(24) | 1,34 | 1,14 - 1,57 | |
| Cervical Cancer(25) | 0,88 | 0,58 - 1,36 | |
| Excess body weight | |||
| Breast Cancer(26) | America (Colombia) | 10,3 | 2,4 - 43,8 |
| Unhealthy Diet | |||
| Breast Cancer(26) | America(Colombia) | 3,1 | 2,6 - 3,8 |
| Alcohol consumption | |||
| Lung Cancer(27) | America (Cuba) | 2,06 | 0,8 - 5,1 |
| Breast Cancer(26) | America (Colombia) | 1,3 | 1,0 - 1,6 |
| Cigarette consumption | |||
| Lung Cancer(27) | América (Cuba) | 8,5 | 3 - 23 |
| Breast Cancers(26) | America (Colombia) | 3,6 | 1,3 - 9,9 |
| UV radiation | |||
| Breast cancer(26) | America (Colombia) | 0,7 | 0,6 - 0,9 |
| Total sun Exposurel | |||
| Melanoma(35) | World | 1,34* | 1,02 - 1,77 |
| Intermittent sun exposure | |||
| Melanoma(35) | World | 1,61* | 1.,31 - 1,99 |
| Helicobacter pylori infection | |||
| Gastric cancer(28) | America (Peru) | 2,36 | 1,36 - 4,04 |
| HPV Infection | |||
| Cervical Cancer(29) | World | 17,47* | 10,45 – 29,22 |
| HBV Infecction | |||
| Gastric Cancer(30) | US | 1,19 | 1,03 - 1,37 |
| Anal Cancer(30) | US | 1,66 | 1,17 - 2,33 |
| Liver cancer(30) | US | 10,6 | 9,66 - 11,6 |
| Intrahepatic duct cancer(30) | US | 1,67 | 1,18 - 2,37 |
| Nasopharyngal Cancer(30) | US | 2,08 | 1,33 - 3,25 |
| Prostate cancer(11) | US | 0,81 | 0,73 - 0,91 |
| HCV infection | |||
| Liver cancer(31) | US | 31,5 | 29 - 34,3 |
| Intrahepatic cancer(31) | US | 3,4 | 2,52 - 4,58 |
| Extrahepatic duct cancer(31) | US | 1,9 | 1,41 - 2,57 |
| Pancreatic cancer(31) | US | 1,23 | 1,09 - 1,40 |
| Anal cancer(31) | US | 1,97 | 1,42 - 2,73 |
| Skin cancer(31) | US | 1,53 | 1,15 - 2,04 |
| Prostate cancer(31) | US | 0,73 | 0,66 - 0,82 |
| Infection by Virus of human inmunodeficiency (VIH) | |||
| Colorectal cancer(31) | US | 1,73 | 1,11 - 2,6 |
| Prostate Cancer(31) | US | 1,65 | 0,98 - 2,79 |
| Breast Cancer(31) | US | 1,5 | 1,01 - 2,24 |
DISCUSIÓN
Cancer is a public health problem, particularly in low and middle- income countries where early diagnostic interventions have low to moderate coverage and in which in many cases preventive interventions are not prioritized. In the case of these countries, among which most Latin American countries are found, important results could be achieved if effective and efficient intervention on the modifiable risk factors, particularly oncogenic infections and risk factors derived from styles of unhealthy life.(6,20).
The attributable fraction studies allow estimating the percentage and number of cancer cases attributable to risk factors that can potentially be avoided, which will allow estimating the savings in lives, in diagnostic expenses, medical care, surgeries, medications, rehabilitation, among others. This, in turn, could convince the health decision- makers of the importance of investing in the intervention on modifiable risk factors which in turn will generate an economic return to the state that can be reinvested to improve the health of the population(4,5,20,37-40). Despite this, no FAP studies are available for cancer at the level of Latin American countries. The main studies come from the United States, Canada, China, England and some Eastern European countries with some methodological differences.
This research shows that the methodological proposal of our "Multidisciplinary Group for Research on Cancer and Lifestyle Medicine", to assess the proportion of cancer attributable to modifiable factors, is feasible and feasible, applicable to Peru and Latin America, and meets criteria similar to published international models.
It is also possible to approximate the relative risks from the meta-analysis that obtain the RR or are approximated by the OR; however, a significant fraction of studies should be considered to come from the United States where the PAF of risk factors linked to unhealthy lifestyles is higher than in countries in Asia and Europe(40). In this way, if a meta-analysis of risk factors for cancer in Latin American countries is available, greater accuracy could be obtained in the PAF estimates. Aspects remain to be investigated for risk factors for cancer in Latin America, particularly those corresponding to oncogenic infections that mainly affect medium and low-income countries.
At the time of the preparation of this article, there was little information available on the population of countries such as Peru regarding the prevalence of exposure to ionizing radiation and ultraviolet radiation, so it is recommended not to include them in FAP studies until information on higher-quality The low frequency of exposure of the population to ionizing radiation means that it is not considered in other FAP studies such as Feng in China(40) and Poirer in Canada (39).
Our Multidisciplinary Group is working on the definitive results of cancer incidence and mortality attributable to these modifiable factors, as well as health economics studies to assess the economic impact in Peru and then elevate it to Latin American countries.
CONCLUSION
In conclusion, it is feasible and viable to conduct FAP studies in Latin American countries, particularly in Peru, where most of the information necessary for its estimation is available. Further studies are required regarding ultraviolet radiation and exposure to ionizing radiation so that they can be considered in future studies.
Authorship contributions: The authors participated in: JADV in the genesis of the idea, JADV, WCRM, WCH, RE in the design of the project, JADV, WCRM, WCH, RE, JLC, AGA, RC, MLA, NG in the collection and interpretation of data, analysis of results, preparation of the manuscript of this research paper.
Financing: Financed by the research fund of Ricardo Palma University, ACUN ° ACU2619-2019.
Conflict of interest: The authors declare no conflict of interest in the publication of this article.
Disclaimer: This manuscript is the sole responsibility of the authors and does not represent an official opinion of Ricardo Palma University, Ministry of Health and the National University of San Marcos.
Received: November 11, 2019
Approved: December 16, 2019
Correspondence: Jhony A. De La Cruz-Vargas
Address:INICIB, Faculty of Human Medicine, Building I-208. 2nd Floor. Avenida Benavides 5440, Surco, Lima-Peru.
Telephone: 708-0000 / Annex: 6016.
Email: jhony.delacruz@urp.edu.pe
BIBLIOGRAPHIC REFERENCES