ORIGINAL ARTICLE
JOURNAL OF THE FACULTY OF HUMAN MEDICINE 2020 - Universidad Ricardo PalmaDOI 10.25176/RFMH.v20i2.2785
COMPARATIVE HISTOPATHOLOGICAL STUDY OF THE PHRENIC NERVE FROM CORPSES WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE AND WITHOUT THIS CONDITION
ESTUDIO HISTOPATOLÓGICO COMPARATIVO DEL NERVIO FRÉNICO PROVENIENTE DE CADÁVERES CON ENFERMEDAD PULMONAR OBSTRUCTIVA CRÓNICA Y SIN ESTA CONDICIÓN
Fernanda Mayara Santos-Santana1,
Byron Martins-Lucena2,
Tales Lyra de Oliveira3,4,
Tácio Nertan Cartaxo de Souza5,
Lysien Ivania-Zambrano6,
Ana Rose de Carvalho-Araujo7,
Ana Lúcia de Gusmão-Freire8
1Faculdade de Medicina, Universidade Federal de Alagoas. Maceió, Brasil.
2Prefeitura de Maceió. Maceió, Brasil.
3Faculdade de Medicina, Universidade Municipal de São Caetano do Sul. São Paulo, Brasil.
4Unidade de Hipertensão, Instituto do Coração, Faculdade de Medicina, Universidade de São Paulo. São Paulo, Brasil.
5Instituto de Ciências Biológicas e da Saúde, Universidade Federal de Alagoas. Maceió, Brasil.
6Departamento de Ciencias Morfológicas, Facultad de Ciencias Médicas. Universidad Nacional Autónoma de Honduras, Tegucigalpa, Honduras.
7Serviço de Verificação de Óbitos, Universidade Estadual de Ciências da Saúde de Alagoas. Maceió, Brasil.
8Faculdade de Fisioterapia, Universidade Estadual de Ciências da Saúde de Alagoas. Maceió, Brasil.
ABSTRACT
Introduction: Functional changes resulting from the evolution of chronic obstructive pulmonary disease (COPD) are progressive and irreversible, causing increased diaphragm work due to pulmonary hyperinflation and airway obstruction. Phrenic nerves have promoted innervation of the diaphragm and may have been compromised in COPD condition. Objective: To compare the morphology of the phrenic nerves of the cadavers with COPD and without COPD by optical microscopy. Methods: An exploratory descriptive studio conducted on the Death Verification Service in Alagoas. Pulmonary and phrenic nerve biopsies will be bilaterally taken from the 38 cadavers after a necropsy with the diagnosis of COPD. Tissue samples were fixed and processed by conventional histology for hematoxylin-eosin (HE) histological slides. Biopsies are divided into experimental groups, one composed by patients with COPD and the other with patients without COPD (control - CTR). This classification was realized after the histological analysis, when typical halls of COPD were found. Histological slides were analyzed by optical microscopy by a pathologist, who was able to assess the study. Results: According to the inclusion and exclusion criteria of the study, if it includes 38 cadavers in the initial evaluation, of which 31 are included in the COPD group and 7 in the CTR group. In the analysis of the phrenic nerves, 8 cadavers, 25.8%, of the COPD group had histopathological changes: perineural edema (75%, n=6), nervous atrophy (12.5%, n=1) and perineural eosinophilic infiltrate (12.5%, n=1). The CTR group does not present histopathological alterations of the phrenic nerves. Conclusions: Given the hallmarks of the biopsies performed on the phrenic nerves of the corpses with COPD, we can infer that there is a tendency for nerve alteration, with perineural edema, to be the major modification found.
Key words: Chronic obstructive pulmonary disease, phrenic nerve, microscopy, autopsy.
RESUMEN
Introducción: Los cambios funcionales resultantes de la evolución de la enfermedad pulmonar obstructiva crónica (EPOC) son progresivos e irreversibles, lo que provoca un aumento del trabajo del diafragma debido a la hiperinflación. Los nervios frénicos promueven la inervación del diafragma y pueden verse comprometidos en la condición de EPOC. Objetivo: Comparar la morfología de los nervios frénicos de los cadáveres con y sin EPOC, mediante microscopía óptica. Métodos: Estudio descriptivo, transversal y comparativo de muestras, tanto de los pulmones como de los nervios frénicos de 38 cadáveres, con diagnóstico de EPOC y sin esta condición.
Las muestras de tejido fueron fijadas y procesadas por histología convencional con láminas histológicas de hematoxilina-eosina. Las muestras fueron compuestas por pacientes con EPOC (EPOC) y sin EPOC (CTR). Esta clasificación se realizó después del análisis histológico. Las láminas histológicas se analizaron mediante microscopía óptica por un patólogo, que fue un evaluador ciego al estudio. Resultados: De acuerdo con los criterios de inclusión, se incluyeron 31 cadáveres en el grupo de EPOC y 7 cadáveres en el grupo CTR. En el análisis de los nervios frénicos, 8 cadáveres, es decir 25.8% del grupo de EPOC, tuvieron cambios histopatológicos como edema perineural (75%, n=6), atrofia nerviosa (12.5%, n=1) e infiltrado eosinofílico perineural (12.5%, n=1). Conclusiones: Los nervios frénicos de los cadáveres con EPOC tienen tendencia a alteraciones histopatológicas, siendo el edema perineural la principal modificación encontrada.
Palabras clave: Enfermedad pulmonar obstructiva crónica, nervio frénico, microscopía, autopsia.
Chronic obstructive pulmonary disease (COPD) is considered the fourth leading cause of death in the world and its progression to 2020 is catastrophic, as it may occupy third place in this ranking(1,2). The main risk factor for COPD is smoking, but other environmental exposures, such as exposure to biomass and air pollution, can contribute. In addition to exposure, some intrinsic factors predispose individuals to develop COPD, such as genetic abnormalities, abnormal lung development, and accelerated aging(3). COPD promotes pulmonary hypersecretion, airway oedema, and bronchial spasms that cause air flow obstruction and increase lung resistance. During the exacerbation of COPD, inspiration becomes superficial and rapid, while exhalation is prolonged, making it impossible to ventilate adequately the air in the lungs, leading to pulmonary hyperinflation(4).
The main respiratory muscle is the diaphragm, which provides pulmonary ventilation by raising the last six pairs of ribs and sternum, increasing the transverse and anteroposterior diameter of the rib cage. And as the exacerbation of COPD becomes frequent, there are changes in ventilatory mechanics, specifically the reduction of hemi diaphragmatic domes, resulting in lower abdominal pressure, which reduces the diaphragm’s ability to generate inspirational pressure and less chest expansion. These changes promote the shortening of ventilatory muscles, creating a mechanical disadvantage to meet the needs of respiratory demand(5).
The functional changes resulting from the evolution of COPD are progressive and irreversible, resulting in a constant increase in ventilatory muscle work due to pulmonary hyperinflation and air flow obstruction, which promotes fatigue and diaphragm exhaustion(6).
The phrenic nerve, which originates from the cervical nerve roots C3-C5, plays an important role in the ventilation process, as it is responsible for the innervation of the diaphragm(6). Peripheral innervation consists basically of nodes and nerves. The phrenic nerve is made up of bundles of fibers surrounded by a layer, called a myelin sheath. The myelin sheath has a diameter of approximately 1 μm, consisting of a lipid membrane rich in glycosylfolides and cholesterol covering axons, facilitating the transmission of nerve impulses(7). Glial cells are numerous in the nervous system and can be classified according to their functional properties and location: Schwann cells, located in the peripheral nervous system (SNP), and oligodendrocytes, located in the central nervous system (CNS). Schwann cells and oligodendrocytes surround axons forming several layers(8).
The myelin sheath acts as an electrical insulator, promoting a greater nerve impulse because it causes depolarization leaps along the axon through the Ranvier nodules. Myelin sheath thickness, axon diameter, quantity and distance between Ranvier nodules,nodal structure and molecular composition of ionic channels influence the conduction speed of nerve impulses which can be up to 100 times faster(7). There is evidence that phrenic nerves may be compromised in COPD due to irreversible changes in ventilatory mechanics, especially due to changes in diaphragm(9,10).
The objective of this study was to compare the morphology of the phrenic nerves of the corpses with COPD and without COPD using optical microscopy.
METHODS
Design
This research was approved by the Committee of Ethics in Research (CER) of the State University of Health Sciences of Alagoas (UNCISAL), Opinion number 1878/2012. An exploratory descriptive study was therefore conducted from April to October 2012. Samples of the lungs and cold nerves of corpses autopsied at the UNCISAL Death Verification Service (DVS), with no diagnosis of COPD. The Informed Consent Form (ICF), attached to the DVS General Protocol, was signed by the family member responsible for the corpse. The samples were divided into two groups: 1) group with COPD (COPD) and 2) group without COPD (CTR). It is noteworthy that the groups were confirmed after histological analysis, respecting the inclusion and exclusion criteria.
The exclusion criteria were corpses with no lobe or lung due to surgery or congenital malformation, who underwent cardiac surgery or who had some specific comorbidities, such as neuromuscular disease, neurodegenerative disease, structural malformation of the thoracic bone, neurological disease and history of trauma (head trauma - TBI or spinal cord trauma - TRM).
Initially, the body information contained in the form completed by the family member in the DVS was confirmed, which noted smoking history as one of the main criteria for inclusion in the COPD group, which would be confirmed by the associated presence of some specific histopathological changes.
Phrenic and pulmonary samples
The material for the analysis was collected using the standard necropsy procedure performed in the DVS, in which a cut was made from the manubrium to the umbilical scar (Figure 1. A) through which the thoracic structures from which it was accessed were accessed. Bilateral samples were taken from the pulmonary apex (Figure 1. B) and from the phrenic nerves, located in the posterolateral regions of the pericardium (Figure 1. C). Pulmonary apex samples were used to confirm the presence or absence of anthracosis, septal thickening and emphysema bullas, which may be results of COPD.
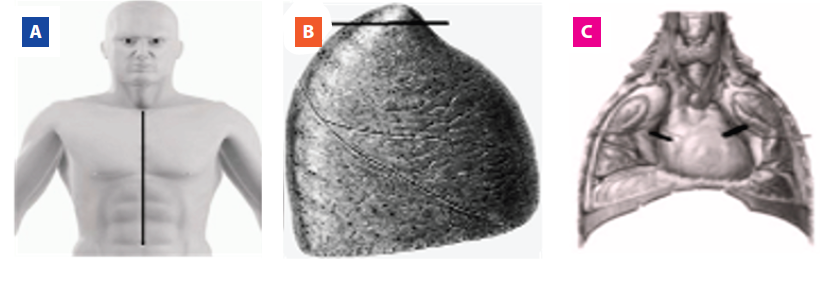
Figure 1. Experimental protocol. A) Section of the region from the manubrium to the umbilical scar to perform the necropsy procedure. B) Section of the pulmonary apex. C) Section of the posterolateral regions of the pericardium for the removal of the phrenic nerves.
Histological processing
The tissue samples were stored in a 10% formaldehyde solution, until conventional histological processing was carried out for the preparation of histological sheets dyed with hematoxylin-eosin (HE). Thus, four histological plates were made for each corpse, one for each pulmonary apex and one for each phrenic nerve. The histological plates were analyzed using optical microscopy with 4x, 10x and 20x lenses. Histopathological analyses were performed by a general pathologist, who was a blind evaluator of the study.
RESULTS
According to the inclusion and exclusion criteria of the study, 38 corpses were included in the initial evaluation, of which 31 were included in the COPD group and 7 in the CTR group. The sociodemographic profile of the samples of the corpses for the CTR and COPD groups can be seen in Table 1. The corpses of the COPD group had an average age of 66.8 ± 17.9 years, higher prevalence of the male gender (61.3%, n=19), mulatto (64.5%, n=20) and originated in the capital (61.3%, n=19). As expected, about 90% (n=28) of the bodies in the COPD group were found to have a history of smoking. While the bodies of the CTR group had an average age of 73.6 ± 17.6 years, higher prevalence of women (85.7%, n=6), mulatto (85.7%, n=6) and origin of the capital (100%, n=7). According to the form completed by the family at the DVS, no corpse included in the CTR group had a history of smoking.
Table 1. Sociodemographic profile of CTR and EPOC body samples.
| CTR (n=7) |
COPD (n=31) |
|
| Gender | ||
| Female | 85.7% | 38.7% |
| Male | 14.3% | 61.3% |
| Age group (years) | 73.6 ± 17.6 | 66.8 ± 17.9 |
| Race | ||
| White | >0% | 32.3% |
| Mulatto | 85.7% | 64.5% |
| Black | 14.3% | 3.2% |
| Procedence | ||
| Capital | 100% | 61.3% |
| Interior | 0% | 38.7% |
| Smoking | ||
| Yes | 0% | 100% |
| No | 100% | 0% |
In lung samples, the most notable histopathological finding was anthracosis present in all samples of the COPD group (Figure 2. B). The COPD group also showed septal thickening and emphysema bullae in 32.3% (n=10) and 22.6% (n=7) of pulmonary samples (Figure 2. C and D, respectively). The CTR group did not present histopathological alterations of lung samples (Figure 2. A).
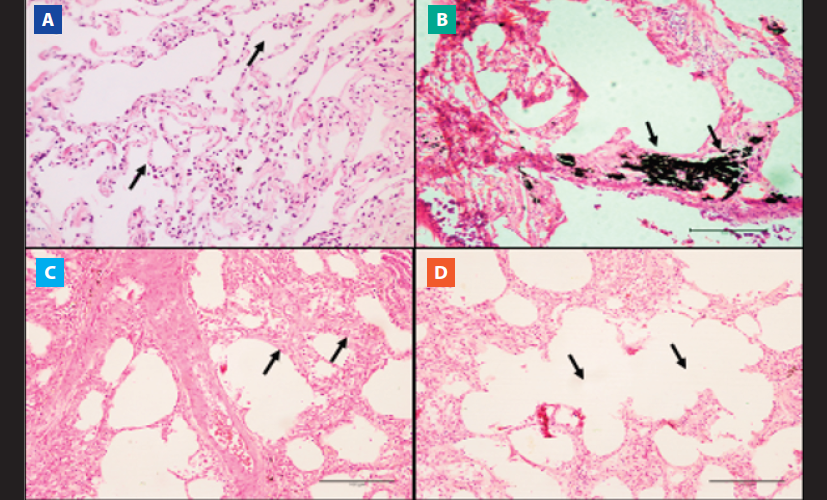
Figure 2. Microscopic view of lung tissue. Images of the histological sections of the pulmonary vertices stained with hematoxylin-eosin (HE) are observed in cadavers with and without COPD. A) Pulmonary morphology with preserved alveolar septa and alveoli (10x). B) Pulmonary anthracosis (10x magnification). C) Emphysematous bubbles. (10x magnification). D) Septal thickening (10x magnification).
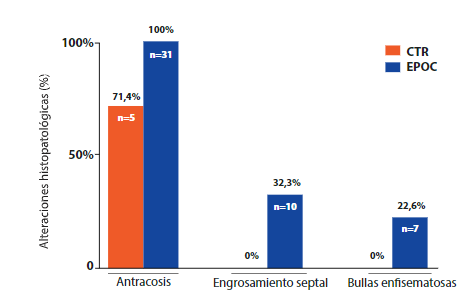
Figure 3. Comparison of histopathological findings according to study group showing pulmonary histopathological alterations in COPD and CTR cadavers.
Regarding the evaluation of the phrenic nerve samples, 8 cadavers, that is, 25.8%, of the COPD group had histopathological changes, such as perineural edema (75%, n=6, Figure 4. B), nerve atrophy (12.5%, n=1, Figure 4. C) and perineural eosinophilic infiltrate (12.5%, n=1, Figure 4. D). The CTR group did not present histopathological alterations of the phrenic nerves (Figure 4. A). Importantly, the histopathological results of the lungs and phrenic nerves presented the pattern bilaterally.
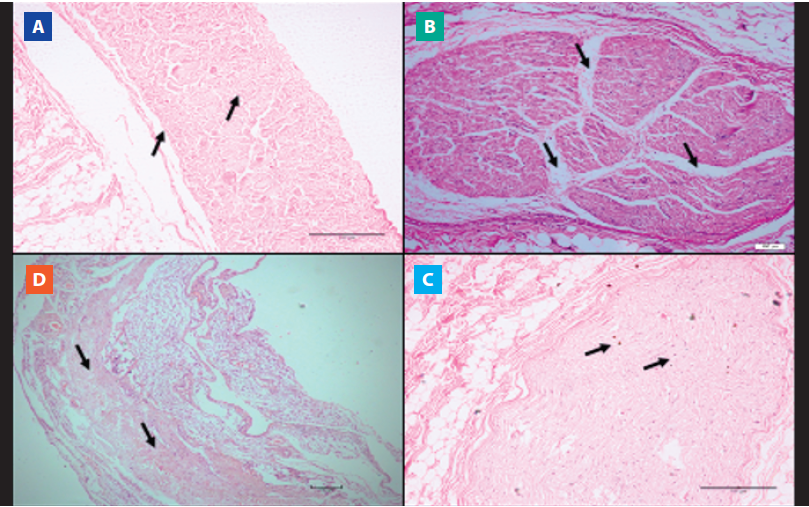
Figure 4. Microscopic view of nervous tissue (phrenic nerve). Histological sheet images with the hematoxylineosin (HE) technique in cadavers with and without COPD. A) Morphology of the preserved phrenic nerve (10x magnification). B) Perineural edema (10x magnification). C) Nervous atrophy (10x magnification). D) Perineural eosinophilic infiltrate (10x magnification).
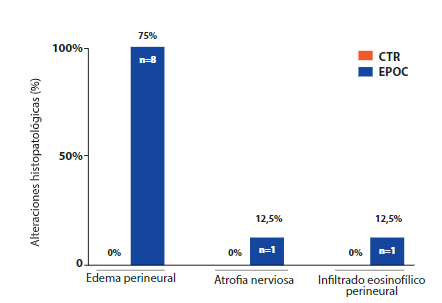
Figure 5. Comparison of histopathological findings according to study group where the histopathological alterations of the phrenic nerves in cadavers with COPD and CTR are seen.
DISCUSSION
Anthracosis was present only in the COPD group. Anthracosis is often found in individuals with a history of smoking, but can also be seen in individuals exposed to biomass burning. The presence of anthracosis in smokers found in this study corroborates data from the literature, confirming that smoking as other factors, such as the combustion of any animal or plant material used as a source of energy, contribute to the appearance of anthracist(11, 12).
Cigarette smoke causes a large and continuous recruitment of inflammatory cells that release proteolytic enzymes in the extracellular matrix of the lung parenchyma, causing characteristic histopathological patterns such as septal thickening, widening of airspaces, reduction of collagen and elastic fibers and increase in the number of alveolar macrophages(13).
Among the histopathological results found in the phrenic nerves of the COPD group, perineural oedema predominated. Studies have shown that perineural oedema can be seen in several neuronal pathologies. The perineural edema provides changes in the neural structure, since it triggers an increase in tissue pressure of the endoneuro, which occludes the arteries and veins and produces ischemia(14, 15).
Nervous tissue is not very tolerant to the ischemic process, so as a compensation mechanism, a thickening of the nerves can occur. If the injured tissue develops fibrosis in the epineuro, the thickening of the nerve will quickly cause an increase in pressure in the internal nervous compartment, initiating a new ischemic process(16, 17). The perineural oedema found in this study is still obscure, because no consistent data were found that directly correlated the perineural oedema with the onset of COPD.
Scott and collaborators found the presence of collagen in the myelin sheath, as well as abnormal muscle fibers in the diaphragms of individuals with COPD, which compromises the generation of strength in this primary respiratory muscle, this reduces the performance of ventilatory mechanics(18). The diaphragmatic adaptation exists simultaneously with the damage to the diaphragm of an individual with COPD, but this adaptation does not guarantee the same strength and resistance that are found in the diaphragm of a healthy individual(9,19,20).
Curiously, studies have found that quadriplegic individuals may have diaphragmatic muscle atrophy after a few months of spinal cord injury, halving the diaphragmatic contractile force, which can be explained by the law of use and desusus(10,21).. From these data, it is possible to infer that motor nerve injury may cause harmful muscle disturbances and, consequently, impaired motor function. Following the same reasoning, the injury of the motor nerve and the consequent denervation of the motor plate promote muscular atrophy, preferably affecting the rapid contraction fibers(22). Therefore, apparently, there is an association between the changes found in the diaphragm and the phrenic nerves, and one event may influence another. It is therefore necessary to elucidate the pathophysiological mechanisms involved in these processes, such as the appearance of muscle alterations and nerve adaptations.
Limitations
As this was a pioneering study, we had difficulties that prevented a detailed analysis of the histopathological changes found in phrenic nerves in COPD. Among them we have:
• The scarcity of existing bibliographic references in the literature, which restricted the elaboration of broader discussions.
• The histological technique used, which was the conventional one.
Therefore, an alternative technique for a better visualization, identification and description of the structures in question, would be the electron microscopy associated with a suitable software for measurements.
CONCLUSION
Given the results of the samples of the present study, the phrenic nerves of the cadavers affected by COPD, presented histopathological changes. It was also observed that these changes in the phrenic nerves may be related to the performance of the diaphragm; however, more studies will be needed to test and explain these pathological events and to learn more about the pathophysiological mechanisms involved in these processes, such as the appearance of muscle disorders and nerve adaptations.
Authorship contributions: The authors participated in the genesis of the idea, project design, data collection and interpretation, analysis of results and preparation of the manuscript of the present research work.
Financing: Self-financed.
Conflict of interest: The authors declare that they have no conflict of interest in the publication of this article.
Received: February 1, 2020
Approved: March 10, 2020
Correspondence: Tales Lyra de Oliveira.
Address: USCS, Rua Treze de Maio, 681, Bela Vista, CEP 01327-000, São Paulo - SP - Brasil.
Telephone: +55 11 95222-4180
E-mail: tales.oliveira@prof.uscs.edu.br
BIBLIOGRAPHIC REFERENCES
