ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i3.2993
VIRAL ETIOLOGY OF THE GUILLAIN-BARRÉ SYNDROME: LOOKING FOR THE IDIOPATHIC ANSWER
ETIOLOGÍA VIRAL EN EL SÍNDROME DE GUILLAIN-BARRÉ: BUSCANDO UNA RESPUESTA A LO IDIOPÁTICO
Jorge Arturo Vega Fernández1,a, Danny Omar Suclupe Campos1,a, Mayra Massely Coico Vega1,a, Franklin Rómulo Aguilar Gamboa2,a
1Department of Microbiology, Faculty of Biological Sciences, Pedro Ruiz Gallo University. Lambayeque, Peru.
2Immunology–Virology Laboratory, Lambayeque Regional Hospital. Lambayeque, Peru.
aBiologist - Microbiologist
ABSTRACT
Guillain-Barré Syndrome (GBS) is a rare disorder of the nervous system, where the patient's immune system attacks the peripheral nerve cells in the arms and legs, causing muscle weakness, loss of sensation and sometimes total paralysis. The origin of this disorder has been associated with immune responses triggered by post-infection with Campylobacter spp. However, when there is no obvious cause of the disease, it is usually not investigated due to the greater interest in the treatment. Therefore, most cases are reported as idiopathic origin. Between January and March 2016 worldwide, GBS outbreaks were reported in 8 countries, linked to the emergence of the Zika virus. In Peru, GBS outbreaks have been reported more frequently since the end of 2018 and, although no association with Zika has been confirmed, the increase in cases, the geographical extension where they occurred and the clinical characteristics of affected patients, have common patterns that lead to suspect an infectious origin mainly of viral type. Therefore, it is important to know the current scientific evidence about the role that some viruses play in this syndrome, allowing us to expand our epidemiological picture with new tools to deal with this disease.
Keywords: Guillain-Barre Syndrome; Viruses; Etiology. (fuente: MeSH NLM).
RESUMEN
El Síndrome de Guillain-Barré (SGB) es un raro trastorno del sistema nervioso, donde el sistema inmunológico del paciente ataca las neuronas periféricas en extremidades, causando debilidad muscular, pérdida de sensibilidad y a veces parálisis total. El origen de este trastorno, ha sido asociado a respuestas inmunes desencadenadas luego de la infección por Campylobacter spp. Sin embargo, cuando no existe una causa evidente de la enfermedad, esta no suele ser investigada debido al mayor interés que requiere el tratamiento. Por ello, la mayoría de los casos se notifican como origen idiopático. Entre Enero y Marzo de 2016 a nivel mundial se registraron brotes del SGB en 8 países, vinculados con la emergencia del virus Zika. En Perú, desde finales de 2018 se ha reportado con mayor frecuencia brotes de SGB y aunque no se ha confirmado asociación con Zika, el incremento de casos, la extensión geográfica donde se produjeron y las características clínicas de los pacientes afectados, tienen patrones comunes que llevan a sospechar un origen infeccioso principalmente de tipo viral. Por lo tanto, es importante conocer la evidencia científica actual acerca del rol que desempeñan algunos virus en este síndrome, permitiendo ampliar nuestro panorama epidemiológico con nuevas herramientas para hacer frente a esta enfermedad.
Palabras Clave: Síndrome de guillain-barré; Virus; Etiología. (fuente: DeCS BIREME).
INTRODUCCIÓN
Guillain-Barré Syndrome (GBS) is defined as an acute polyradiculoneuropathy, characterized by a flaccid, ascending, and symmetric paralysis of the limbs, rapidly progressive with hyporeflexia or areflexia, which may be associated with sensory disturbances and cranial nerve deficits in some patients. It occurs more frequently in young adults, with men being more affected than women (3:2 ratio) (1,2). With the advancement of medical research, the term GBS began to be applied to a broad spectrum of acquired acute inflammatory polyradiculoneuropathies, immunologically mediated and presumably triggered by a previous infection (post-infectious), with various pathophysiological mechanisms (demyelination, motor or sensory axonal injury -motor) (3,4).
Between January and March 2016, GBS outbreaks were reported worldwide in 8 countries(1), linked to the emergence of Zika virus as a concomitant infection in many cases reported in the Region of the Americas(5). Likewise, at the end of the summer of 2018 and autumn of 2019, the north and center of Peru had reported a disproportionate increase in GBS cases, and due to the geographical extension where they occurred and the clinical characteristics of the affected patients, etiology was suggested. viral, with Zika and Enterovirus D68 being the main suspects(6). However, a large number of cases do not identify the infectious agents and are more likely to end up for epidemiological registry purposes as cases of idiopathic origin. It is necessary to broaden our epidemiological panorama considering other probable etiological agents such as viruses, which are also widely recognized in the scientific literature but whose participation is usually little studied and dismissed, either due to lack of resources, complexity in their difficulty of detection, or simply due to the interpretation of serological and molecular tests.
This article brings together current information on the recognized and probable associations between viral infections and GBS that should be taken into account given the increase in cases recorded in recent years in the Region of the Americas. Likewise, clinical laboratory tools are described that allow their adequate investigation and interpretation, since this is usually a frequent obstacle that motivates the under-reporting of microorganisms, mainly viruses, as part of the probable etiology of this syndrome.
METHODOLOGY
In this article, everything related to GBS and its viral etiology has been reviewed, using the MEDLINE accessed from PubMed, Web of Science, Scopus, Scielo, Virtual Health Library (VHL). The search was performed in isolation or a combination of these keywords "Guillain-Barré syndrome" and its combinations with "virus/etiology". Original articles, reviews, case reports or case series, and information obtained from web pages that develop the central theme of the article were identified.
PATHOPHYSIOLOGY OF GBS
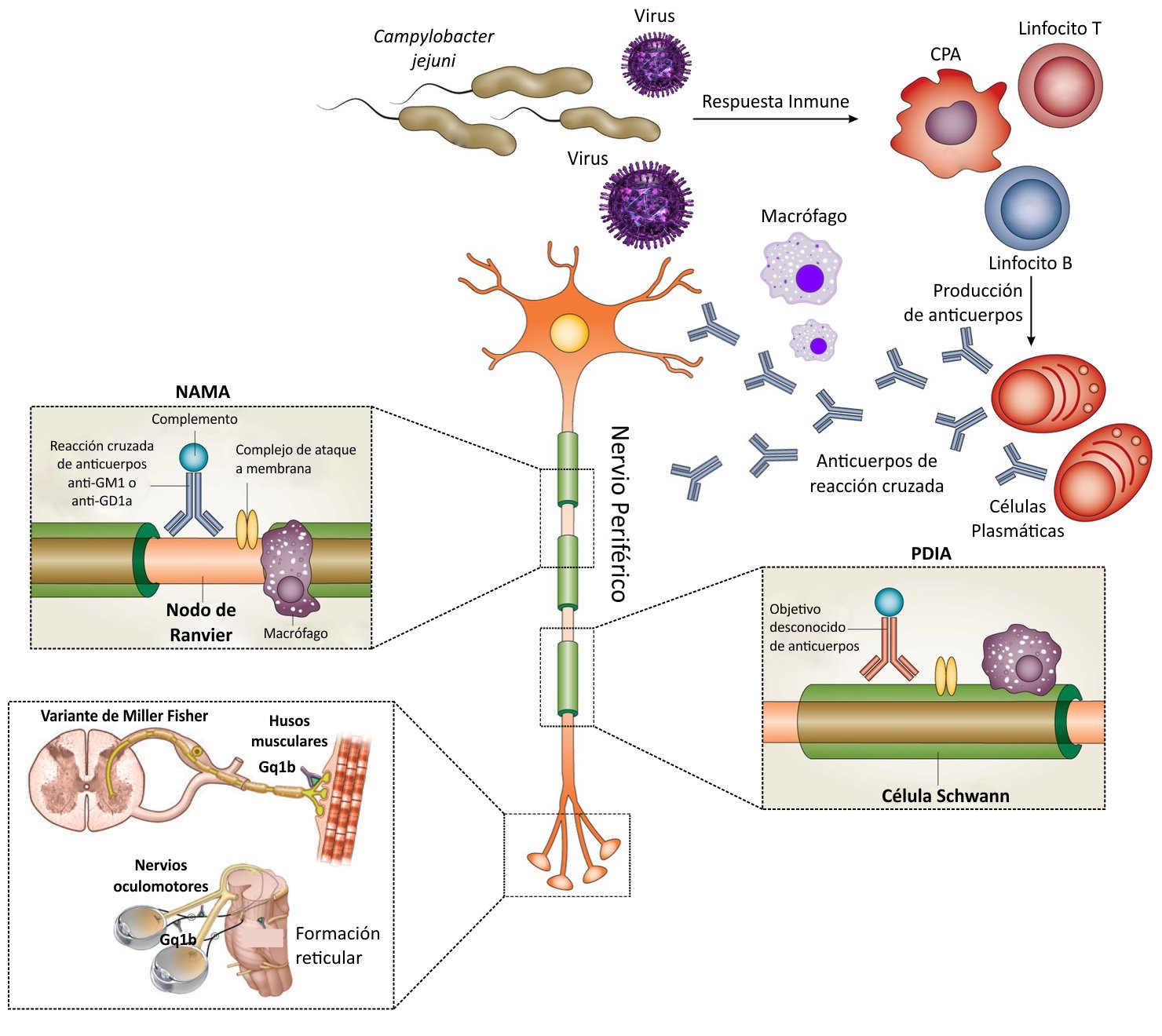
Different variants or subtypes of GBS have been described according to their clinical and neurophysiological characteristics, including acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AAM), acute sensory-motor axonal neuropathy (NASMA), and Miller-Fisher Syndrome (MFS)(3). The different subtypes are mediated by immune mechanisms that involve autoantibodies causing structural and functional damage mainly to the myelin and axons of the peripheral nervous system (PNS) (4,7). The immunopathogenesis differs in each of them; however, those involving cells and antibodies against myelin constituents of the PNS (PDIA) can be grouped together; and to subtypes of fundamentally axonal commitment (NAMA, NASMA and SMF) (Figure 1) (3,8). Although both elements of the immune response (T and B lymphocytes) play an important role, current understanding holds that GBS is mediated by antibodies that damage the myelin membrane and axons of the PNS, due to cross-reactivity with bacterial epitopes and/or viral by molecular mimicry (9,10).

The most common form of GBS is PDIA, which arises from demyelination of the peripheral nerve, mediated by antibodies, macrophages, and complement deposition in the myelin membranes of Schwann cells (Figure 2). The demyelination process is generalized and affects most of the myelinated limbs, and lower axial cranial motor and sensory nerves, but curiously it does not affect myelinating axons that innervate extraocular muscles that are affected in FMS (11).
In contrast to PDIA, the main target for attack by the immune system in the NAMA and NASMA variants is the axolemmal membrane. This inflammatory process occurs predominantly in either the nerve roots or distal nerve terminals. (Figure 1). The immune attack can lead to conduction block by reversible axonal injury at best or complete axonal transaction at worst. Wallerian degeneration (disruption of the axon and its myelin sheath after injury to the proximal part of the axon or the neuronal soma) is a typical event in these variants of GBS (11).
Gangliosides, carbohydrate residues linked to a lipid fraction, are found forming a superficial part of cell membranes in the peripheral nervous system (12,13). Approximately 188 types have been characterized in vertebrates (14) and they constitute specific classic molecular targets of antiganglioside antibodies (AGA) present in this disease. Molecular mimicry between infectious agents and gangliosides plays an important role in the induction of these antibodies that contribute to peripheral nerve inflammation-demyelination processes in patients with GBS (15). Various studies show that lipooligosaccharides (LOS) from infectious organisms contain epitopes that are similar to gangliosides in peripheral nerves, for example, in Campylobacter jejuni, LOS mimics the membrane gangliosides GM1, GM1b, GD1a, and GalNAc-GD1a very well. and anti-LOS antibodies produced against infection have cross-reactivity with antiganglioside antibodies resulting in injury to peripheral nerve fibers (Figure 2)(15). Similarly, in patients with NAMA, antibodies against C. jejuni and H. influenzae are cross-reactive with IgG immunoglobulin subclasses IgG1 and IgG3 that bind to gangliosides, producing complement-dependent axonal damage (15). In animal models, these axonal injuries are due to complement fixation with macrophage recruitment and attack complex deposition (Figure 2) (16). This immunological cascade disrupts the anatomical and physiological integrity of the exposed membranes in the nerve terminals and nodes of Ranvier, causing nerve conduction block. In patients with FMS, oculomotor weakness has been associated with anti-GQ1b antibodies, where the ganglioside GQ1b is the antigenic target in the nerves that innervate the extraocular muscles (Figure 2) (7,11).
Unlike NAMA, the immune cascade involved in PDIA is less well understood for several reasons. First, a broader range of immune stimulants causes PDIA, including bacterial and viral infections, and post-vaccination reactions. Second, the specific antibody biomarkers have yet to be characterized. Alternatively, nerve-specific T cells, directed against yet unknown antigens, might play a more important role in PDIA than is currently known (Figure 2) (11).
PROBABLE ROLE OF VIRUSES IN CAUSING GUILLAIN BARRÉ SYNDROME
Cytomegalovirus and antiganglioside antibodies as evidence of the autoimmune factor in GBS.
Cytomegalovirus (CMV) infection is very common and has a worldwide distribution. It occurs at any age, with a higher incidence during the first years of life. CMV is the main cause of congenital viral infection, and it is estimated that the infection is present in 0.5-1% of all newborns, of whom approximately 10% will develop symptoms (17). Multiple complications (1.7-5.2%) of varying severity have been described, with neurological origins such as encephalitis, meningitis, and GBS is very common (17).
Since the late 1960s, the association between CMV and GBS has been known. Studies have shown that around 12-13% of patients with GBS had a previous CMV infection(18). It is currently considered the most common viral infection associated with this syndrome, being identified in up to 15% of patients by detection of specific IgM antibodies and in 62% of patients by DNA detection(19). It is estimated that the risk of GBS after CMV infection can reach 0.6-2.2 cases per 1,000 people(20). Likewise, it has been suggested that molecular mimicry with neuronal structures in primary CMV infection is more likely than in the course of virus reactivation, due to lower antibody specificity and affinity early after infection(19). On the other hand, GBS occurs sporadically in kidney transplant patients, in whom the use of oral antiviral drugs in combination with immunoglobulins and plasmapheresis reports relative success(21), however, analytical studies and clinical trials are lacking. regard.
In childhood, GBS after CMV infections is rarely reported and if it does occur, it often begins with severe symptoms and longer recovery times. However, the sensory-motor axonal form of the syndrome after CMV infection has a good outcome prognosis with prompt and complete recovery in children(22).
In cases that report an association between CMV and GBS, both positive anti-CMV and anti-GM2 IgM(22), some studies even describe increases inanti-GM2 IgM, anti-GalNAc-GD1a, and anti-GalNAc-GD1a antibodies. myosin, a protein that exists in trace amounts at the nodes of Ranvier. However, these are no longer detectable 4 months after therapy(23). It is important to take into account the serological study in search of these markers during GBS, because the probable association of antiganglioside antibodies (AGA) against anti-CMV antibodies during this disease becomes increasingly relevant( 24).
The expansion of the Zika virus in America and its probable relationship with GBS
The virus was first isolated in the Zika forest of Uganda in Africa around the 1950s, and reappeared in 2007, with important reports of fever taking place on the island of Yap, in the western Pacific (Micronesia) (25). Years later, French Polynesia was first affected by ZIKV (26) and although in recent years the risk of microcephaly and other congenital brain anomalies were the main complications associated with the virus (25), during the outbreak of October 2013 and April 2014, a study reported that of 42 patients with GBS, 37 (88 %) developed a viral syndrome compatible with ZIKV at a median of 6 days before the development of neurological symptoms. The characteristics and evolution corresponded to the NAMA subtype of GBS (27,28). Likewise, in 2016, Colombia registered 68 patients with GBS, of which 97% had clinical symptoms compatible with ZIKV infection, finding both virus RNA and antiflavivirus antibodies in 40 and 43% of patients. It was even suggested that GBS be reconsidered as a parainfectious complication, instead of a postinfectious one (28,29). However, we must take into account that this probable relationship was suggested based on descriptive studies where GBS cases were reported during a Zika outbreak evaluating parameters such as frequency and incidence, but there are no conclusive studies in this regard, being necessary to carry out analytical studies to determine the association between this arbovirosis with different neurological alterations such as GBS(30).
The Antibody-Dependent Enhancement Model (ADE) of the Dengue virus and its relationship with GBS
The Dengue virus represents the most important arbovirus that currently affects humans, both in the febrile syndrome and in the severe type, it can include neurological signs such as acute transverse myelitis, acute disseminated encephalomyelitis, and GBS(31). Although most reports do not describe demyelination as a specific complication(32,33), there are case series reports that relate it to GBS(31) and others that relate the NASMA variant as a possible neurological complication by DENV(34).
Hemorrhagic fever and Dengue shock syndrome arise through immunopathological mechanisms following sequential infection of an individual with these antigenically related heterologous serotypes. "Immune potentiation" is believed to play an important role in pathogenesis. Several antigenic determinants for infection-enhancing antibodies have been found on the envelope glycoprotein. Therefore, the same cross-reacting antibodies from a previous infection could act against antigenic determinants leading to demyelination under an antibody-dependent enhancement (ADE) model (32,35). However, regarding the nervous manifestations, there is great controversy about whether the neurological signs associated with DENV infection are due to direct infection in the nervous tissue, or if they are the result of nerve dysfunction associated with damage. or failures in extraneural organs, as in the case of hepatic encephalopathy, or the presence and constant circulation of systemic inflammatory mediators or metabolites increased by the infection, which modulate neurological function. To elucidate this, complete clinical and paraclinical analyzes and the use of complex imaging would be required, however, these are almost never done (36).
The Epstein-Barr Virus precedes GBS mainly in children
The Epstein-Barr virus (EBV) can present neurological manifestations that include encephalitis, aseptic meningitis, and even GBS, most of which have been reported mainly in children (37–39). Studies have shown that up to 90% of patients who developed GBS may have high IgG titers for EBV and anti-GM1 and GM3 antibodies (40).
The presence of EBV infection in GBS is relatively rare, with a related incidence rate of 0.36% and although the evolution in children is frequently complete, it seems that this would not be the case in children under 2 years of age (41). FMS has been associated with primary EBV infections, both in children and adults, where anti-GQ1b antiganglioside antibodies were found in 90% of FMS cases and positive serology for VCA and EBNA, as well as the presence of the virus in plasma. which suggests a late primary infection (42). However, it is important to properly interpret these types of events because the presence of antibodies against EBNA would indicate an infection approximately 3 months earlier, making the participation of this virus in GBS unlikely due to being an event that is too late. A true association between the virus and the syndrome should be determined by the presence of IgM antibodies against VCA in the absence of EBNA antibodies, which would reveal a very recent infection and help elucidate the cause of the disease.
Varicella-Zoster Virus infection is associated with a demyelinating subtype of GBS
Varicella Zoster virus (VZV) belongs to the Herpesviridae family, which is known to have a tropism for the peripheral nervous system. Neurological complications are reported after infection by this virus. Of these, encephalitis is the most common (1: 1,000) people (43) and GBS the least common (1: 15,000) people(44). Less than 50% of these cases have been reported in the literature and, when they occur, it is almost always due to the reactivation of latent VZV in Herpes zoster (shingles) (43). Nevertheless, In Bangladesh, the frequency of GBS preceded by primary infection by VZV has been described. Between 2010 and 2016, of 536 patients, only 1.3% (7 patients) had chickenpox in the 4 weeks before the onset of GBS. Anti-VZV IgM antibodies were present and anti-GM1 were negative in all cases. VZV infection is associated with the demyelinating subtype of GBS, clearly distinct from the axonal form of GBS that predominates in this country (45). However, an alternative mode of pathogenesis in PDIA, which is currently poorly understood, has been suggested elsewhere(43).
GBS usually occurs in the early stages of infection by the Human Immunodeficiency Virus (HIV)
Neurological involvement in HIV-infected patients is common and involves the central and peripheral nervous systems. It has been suggested that 30% of these present neurological involvement as the first manifestation of HIV infection and it is recognized as the cause of death in 11% of them (46,47).
In 1985, the association between GBS and HIV in a patient with AIDS was described for the first time. Three GBS patients were then described, at the onset of HIV infection, before AIDS(48). Currently, GBS in HIV-infected patients is considered to precede overt AIDS and evidence of immunosuppression (48,49). A study of 32 GBS patients in Zimbabwe found that 16 (55%) had HIV infection (50). Although some patients had AIDS and GBS, it has been suggested that GBS occurs early in HIV infection with high blood counts. CD4 lymphocytes (> 500) (48,49). In Tanzania, this association has been confirmed, with a prevalence of 30.5% (11 of 36 patients) of the virus in patients with GBS. GBS seropositive patients had a shorter duration of onset, greater neurological involvement, and a higher mortality rate 45.5% (5/11) versus 16% (4/21) compared with seronegatives (50, 51).
In recent years, the role of HIV has become so important that some researchers refer to the need for patients with symptoms of GBS, regardless of clinical history, to be offered an HIV test. Because the syndrome may be the first sign that a patient is HIV positive (52) and although GBS usually occurs mainly before the AIDS phase, it should be considered that in advanced stages concomitant infections with other viruses, especially CMV, could develop the disease(46) .
Post-exposure GBS to the Influenza virus and the probable effect of its vaccine
Neurological manifestations are an important complication of Influenza infection. A wide variety has been reported, of which febrile seizures and encephalopathy are the most common, with approximately three-quarters of cases occurring in children (53,54). A French study determined that of 73 patients with GBS of unidentified cause, 13.7% had serological evidence of Influenza A virus, while 5.5% had Influenza B (55). Likewise, one study found an increase in GBS two months after infection associated with similar symptoms of Influenza or acute respiratory infection. However, GBS has been reported sporadically in cases of Influenza 2009 AH1N1 (56). Likewise, in a Norwegian study of 490 GBS cases during 2009-2012, 410 occurred after October 1, 2009, of which 46 new cases occurred during the peak period of the influenza pandemic. This indicated that there was a significantly increased risk of GBS during and after pandemic influenza infection (57).
On the other hand, there are also reports of GBS after exposure to the influenza vaccine. In 1976, the United States began a massive immunization program against the A/NJ/76 strain of Influenza H1N1, with 40 million vaccines distributed, after which a total of 532 cases of post-vaccination GBS were reported (58). It has been proposed that the viral compounds of the vaccine, under certain conditions, could result in a structure that mimics ganglioside epitopes and thereby explain molecular mimicry (56).
Hepatitis viruses and GBS
There is evidence that hepatitis viruses, especially hepatitis A as an antecedent to the development of GBS, as for this virus, there is enough literature that indicates it as a frequent complication, which would be related mainly to cases arising in young adults between 21 and 34 years old (59–62); it should be noted that no report found that children were affected after infection by this virus. Regarding hepatitis E (HEV), it is known that it can cause extrahepatic manifestations.
Several cases have been reported with HEV infections associated with neurological disorders, including GBS, brachial neuritis, and polyradiculopathy. Interestingly, one-third of GBS patients show mild liver function abnormalities with no obvious cause. (63.64). Some reports have found an association of up to 5% of patients with GBS who recently had an infection by (HEV) with high levels of IgM anti-HEV antibodies, however, antiganglioside antibodies were not detected (65). Therefore, their association is unlikely. As for hepatitis B and C viruses, it is known that the former has rarely been implicated in acute polyneuropathies mediated by the immune system that are classified under the term GBS; These associations have represented approximately 1% of GBS cases, and their report is very rare. (66). As for the second, it should be examined in high-risk patients to prevent the silent progression of chronic hepatitis C and its potentially serious extrahepatic manifestations, which in rare cases also includes GBS. (67).
EnterovirusD68: a distinct cause of flaccid paralysis
Enteroviruses (EVs) are a large group of viruses, including Coxsackieviruses, Echoviruses, Enteroviruses 68-71, and Polioviruses. Humans are the only natural host. The infection is highly contagious by direct contact and then enters the CNS, although it cannot be determined whether this occurs during secondary viremia or by upward migration through peripheral nerves. Asymptomatic and minor infections are more frequent than the paralytic forms, with a ratio ≥ 60:1, being the main source of spread of the disease (68).
Enterovirus D68 can cause a wide range of respiratory disorders in children, from pharyngitis and bronchitis to more severe pneumonia and respiratory failure. Healthy adults can also become infected with Enterovirus D68 (EV D68), although it usually presents with a milder range of respiratory symptoms (68). However, EV D68 is strongly associated, both in children and adults, with acute flaccid myelitis. (MFA) (69), which is a complex syndrome characterized by the sudden onset of weakness in one or more limbs or in the respiratory and bulbar muscles as a result of lower motor neuron damage (70). Some reports from Peru indicated the implication of EV D68 in the development of GBS, however, there is no evidence to support it as a probable cause of the syndrome, a fact that was widely discussed by a study on the subject (71).
Human parvovirus B19 and heterogeneous evidence as antecedents to GBS
The last 10 years have witnessed an increase in cases of GBS associated with human Parvovirus B19 (PVB19). Data in the literature on the incidence of neurological manifestations remain scattered, and heterogeneous, and epidemiological information cannot be accurately extrapolated (72). However, there are neuropathies very similar to GBS and its associated variants (PVB19), more frequently in children after developing erythema infectiosum (fifth disease). Symptoms include muscle weakness of the lower extremities with mild sensory disturbances, areflexia in some cases, and others with myelinated and non-myelinated axonal degeneration, compatible with acute motor sensory neuropathy (73). FMS has been reported in some pediatric cases with facial diplegia, and abnormal demyelination with alterations in motor and sensory nerve conduction. However, without antiganglioside antibodies, which would make us doubt a molecular mimicry behind this event (74). An atypical SMF called anti-GQ1b syndrome with unilateral acute paralysis of the third cranial nerve has even been reported (75). However, in none of these cases was GBS specifically mentioned.
DISCUSSION
GBS is an inflammatory disease of the peripheral nervous system and is the most common cause of acute flaccid paralysis. In Peru, between 2012 and 2017, 955 cases of GBS were reported, with a higher frequency in men and the population aged 20 to 59 years. The national incidence of GBS per 100,000 inhabitants was 0.91 in 2017, being higher in older adults and men. Likewise, the overall fatality rate was 3.5%, which was higher in those older than 60 years. However, in 2018 and 2019 the number of cases experienced a dramatic increase, mainly on the north coast of the country, leading to suspicion of a post-infectious origin, as happened in the Zika virus epidemics in French Polynesia in 2013 and in Latin America and the Caribbean in 2015 - 2016 where the number of GBS cases also followed this pattern (76).
In recent years there have been major outbreaks of the disease and in this context, the search for an etiological agent becomes more and more justifiable because, despite its autoimmune nature, the seasonal patterns, the number of cases, and the previous clinical characteristics suggest the presence of an infectious agent involved. Regarding this, in 2019 some reports in Peru indicated that Enterovirus D68 would have some relationship with GBS. However, the latter was widely disputed (77).
The role of some viruses in the development of GBS is increasingly evident. Such is the case of CMV, whose relationship has been known since the 1960s. Such is the evidence that supports it that analytical design studies have been able to calculate the risks of suffering from GBS against infection by this virus (20). Likewise, some studies have managed to relate it to the presence of antiganglioside antibodies (24). In the same way, the Zika virus during 2013 was presented as an important cause of GBS and although observational studies supported this fact, there could be a selection bias in the sense that all the evidence regarding these cases arose during the development of a Zika virus outbreak.
When analyzing the role of the Varicella Zoster virus in the development of the disease, it does not seem to be so relevant. In a 2019 study, of all the infectious agents reported in patients diagnosed with GBS, antibodies against this virus were found in only 1% of them (78). This low frequency would be attributed to the fact that the number of chickenpox cases has decreased due to the vaccine and, although it occurs sporadically, in some cases of GBS, it is important to consider the role of this virus as a possible etiological agent (79).
In other cases, although the evidence is consistent, their lower frequency makes it difficult to assess their real impact on the disease, such as what happened in dengue virus infection, in which, apparently, the development of GBS it depends on the phenomenon of antibody-dependent infection amplification (ADE) that occurs after second or third infections with the virus. Regarding EBV, although there are reports on the matter, it is difficult to adequately interpret the serological studies presented by patients with GBS. For which the absence of markers against EBNA and the presence of VCA should be considered, which would be evidence of a recent infection. Regarding HIV, the current evidence emphasizes that tests to rule out GBS patients should be offered, since this syndrome can often be the first sign of infection by this virus (52).
Both vaccination and viral infection due to Influenza are reported as a risk of developing GBS. However, it should be considered that when evaluating the incidence rate of GBS in the Norwegian population during the peak of the 2009 pandemic in relation to other periods, a 1.46 [95% confidence interval (CI) (1, 08-1.98). Likewise, the adjusted hazard ratio (HR) of GBS within 42 days after diagnosis of pandemic influenza was 4.89 (95% CI 1.17-20.36). After pandemic vaccination, the adjusted HR was 1.11 (95% CI 0.51 to 2.43). All of these data revealed that there was a significantly increased risk of GBS during the pandemic season and after pandemic influenza infection. However, vaccination did not increase the risk of developing the disease (57). Regarding this topic, a meta-analysis evaluated the possible adverse effect of influenza vaccines on the production of GBS, in which it was emphasized that this probably should not negatively affect its acceptance due to the very little relevant information that exists in this regard. , highlighting the lack of continuous monitoring of the safety of influenza vaccines (80).
The viruses mentioned above are characterized by being enveloped, thus, we could suspect that only enveloped viruses would produce GBS, which would be supported by the presence of gangliosides in the structure that forms it and that would be responsible for molecular mimicry. However, the hepatitis A virus, a naked virus, presents robust evidence of being involved in the production of GBS. The role of this virus in GBS would be explained by the fact that despite lacking an envelope, it has a recently described lipid cover (81), which could present gangliosides responsible for molecular mimicry. Other viruses mentioned as antecedents of GBS, which have been reported in sporadic cases, are the rubella virus and the recent SARS-CoV-2, of which, although an important role in neurological damage has been reported, it is not yet known if participate in GBS (82).
The fact of finding articles that jointly report neuropathies such as GBS and infectious agents in situ (83); motivates questioning the role played by the latter in certain pathologies. In this sense, it is known that neuropathies are generally divided into post-infectious and para-infectious categories. The first is caused by autoimmune reactions to the infectious agent, which cross-react with neural antigens from Schwann cells/myelin or axons in the peripheral nerve. As occurs in GBS, in which polyneuropathy usually develops several weeks after infection. As for the second, parainfectious neuropathies usually develop during or shortly after an acute infection. These develop as a direct consequence of infection or as an unusual hyperimmune response. Infectious agents that cause neuropathies by this mechanism include Borrelia species, Brucella, Clostridium botulinum, and West Nile virus, which can cause polio-like illness (84). In this regard, according to the pathophysiology of the development of GBS described above (Figure 2), there is no evidence that this syndrome is parainfectious, therefore, any isolation of infectious agent concomitant with the development of the syndrome should not be considered as a probable causal agent (77).
The most appropriate studies to investigate the role of viruses in GBS should be oriented to the search for antibodies, due to the post-infectious nature of the disease. Studies that look for a virus in situ would represent recognizing its parainfectious nature; however, this approach is far from being the most appropriate due to the above. Serological studies focused on the search for etiological agents of GBS are very few, one of the latest on this topic reports influenza A and B, hepatitis A virus, DENV, CMV, and EBV as important antecedents of GBS after Campylobacter spp (78).
CONCLUSION
Given the available scientific evidence, the role played by viruses in the etiology of GBS is undeniable. Although Campylobacter spp. It is still the main agent involved in this syndrome, many viruses such as Influenza A, CMV, Zika, among others, are also involved in its origin. Apparently, an important and necessary condition for these infectious agents to produce GBS would be the presence of an envelope, which would favor molecular mimicry, mainly with antiganglioside antibodies that would subsequently trigger the disease. Therefore, the role of naked viruses in the production of GBS would be unlikely. The post-infectious nature of the disease should be considered, and not guide the search for etiological agents in situ, which for many years would have contributed to reporting the cause of this syndrome as idiopathic as there was no reportable finding. A suitable tool for this purpose would be the serological study since the presence of antibodies would reveal infectious events that occurred previously. Finally, it is important to recognize viruses as the causative agents of GBS, especially when outbreaks of this disease occur, which, due to the demographic and clinical pattern presented by patients, make it necessary to think that an infectious agent is circulating. A fact that should reorient containment measures against this disease.
Authorship contributions: Jorge Arturo Vega Fernández: Conception of the original idea, collection of information, writing of the article, critical review of the article, contribution in the elaboration of figures and approval of the final version.
Danny Omar Suclupe Campos: Collection of information, writing of the article, critical review of the article, approval of the final version.
Mayra Massely Coico Vega: Collection of information, approval of the final version.
Franklin Rómulo Aguilar Gamboa: Collection of information, writing of the article, critical review of the article, approval of the final version.
Funding Source: Self.
Conflict of interest: Los autores declaran no presentar conflicto de intereses.
Submitted: 28 May 2020
Accepted: 07 June 2022
Correspondence: Franklin Rómulo Aguilar-Gamboa.
Address: Pro. Augusto B. Leguía Nro.100 (Esquina Con Av. Progreso N. 110-120), Chiclayo-Perú.
Telephone number: 971339765
E-mail: faguilar@hrlamb.gob.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
REFERENCES
