ORIGINAL PAPER
JOURNAL OF THE FACULTY OF HUMAN MEDICINE 2020 - Universidad Ricardo PalmaDOI 10.25176/RFMH.v20i3.3040
CLINICAL-PATHOLOGICAL CHARACTERIZATION, VIRAL GENOTIPIFICATION AND GENETIC HETEROGENEITY AS RISK DETERMINANTS IN COVID-19: STUDY DESIGN AND INITIAL FINDINGS
CARACTERIZACIÓN CLÍNICOPATOLÓGICA, GENOTIPIFICACIÓN VIRAL Y HETEROGENEIDAD GENÉTICA COMO DETERMINANTES DE RIESGO EN COVID-19: DISEÑO DEL ESTUDIO Y HALLAZGOS INICIALES
Mauricio Postigo Mac Dowall1, Alejandro Barrionuevo-Poquet1, Oscar Carnero-Fuentes1
, Guido Pareja-Begazo1
, Claudio Coayla-Cano1, Aly Gallo-Lopez 2, Jhony A. De La Cruz-Vargas2
1Hospital Base Carlos Alberto Seguín Escobedo-EsSalud, Arequipa-Perú.
2Instituto de Investigación en Ciencias Biomédicas, Universidad Ricardo Palma, Lima-Perú.
ABSTRACT
Introduction: The objective of the study is to describe the clinical, pathological, virological and genetic characteristics of the immune response of patients diagnosed with SARS-CoV-2 infection and its relationship with the unfavorable
course of the disease. Methods: Descriptive, relational, longitudinal and retrospective study based on the review of medical records, taking post-mortem tru-cut biopsies of the lung and liver, taking blood samples and naso-oropharyngeal
swab or endotracheal tube aspirate. In the first phase, the biopsies will be processed and studied with conventional and immunohistochemical histology in the Pathological Anatomy service of the Carlos Seguín Escobedo National Hospital in Arequipa,
Peru. Results: Advanced mean age, male sex, and the presence of comorbidities were predominant in deceased patients. Lung biopsies showed predominantly the exudative and partially proliferative phases of diffuse and focal alveolar
damage, and acute organizational and fibrinoid pneumonia, associated primarily with intraalveolar macrophage hyperplasia with accumulation within the alveolar space-resembling desquamative pneumonia, as well as binucleated intraalveolar pneumocytes
and atypical, with eosinophilic nucleoli (similar to virocytes) in some cases. In the vast majority of cases, intravascular fibrin deposits associated with the accumulation of inflammatory cells composed of neutrophils and monocytes, representing
microthrombosis, were observed. Liver biopsies showed predominantly macrovesicular steatosis and in two cases microvesicular steatosis was observed. Additionally, varying degrees of necrosis and mild portal and lobular inflammation were observed.
Conclusion: The clinical and pathological findings in this first report are consistent with previous publications and confirm the pattern of diffuse alveolar damage associated with aggregates of intraalveolar macrophages and microthrombosis;
in addition, macro and microvesicular hepatocytic steatosis, together with variable degrees of necrosis.
Key words: SARS-COV-2; COVID-19; Pathology, Immunohistochemistry (source: MeSH NLM).
RESUMEN
Introducción: El objetivo del estudio es describir las características clínicas, patológicas, virológicas y genéticas de la respuesta inmune de los pacientes diagnosticados con infección por SARS-CoV-2 y su relación con el curso desfavorable
de la enfermedad. Métodos: Estudio descriptivo, relacional, longitudinal y retrospectivo basado en la revisión de historias clínicas, toma de biopsias tru-cut post-mortem de pulmón e hígado, toma de muestras de sangre e hisopado naso-orofaríngeo
o de aspirado del tubo endotraqueal. En la primera fase las biopsias serán procesadas y estudiadas con histología convencional e inmunohistoquímica en el servicio de Anatomía Patológica del hospital Nacional Carlos Seguín Escobedo de Arequipa,
Perú. Resultados: La edad media avanzada, el sexo masculino y la presencia de comorbilidades fue predominante en los pacientes fallecidos. Las biopsias pulmonares mostraron predominantemente las fases exudativa y parcialmente proliferativa
del daño alveolar difuso y focal, y neumonía organizativa y fibrinoide aguda, asociada principalmente a una hiperplasia de macrófagos intraalveolares con acumulación dentro del espacio alveolar, semejando una neumonía descamativa, así como
neumocitos intraalveolares binucleados y atípicos, con nucléolos eosinofílicos (semejante a virocitos) en algunos casos. En la gran mayoría de casos se observaron depósitos de fibrina intravascular asociada al acúmulo de células inflamatorias
compuestas por neutrófilos y monocitos, representando microtrombosis. Las biopsias de hígado mostraron esteatosis predominantemente macrovesicular y en dos casos se observó esteatosis microvesicular. Adicionalmente, se observaron diversos
grados de necrosis e inflamación portal y lobular leve. Conclusión: Los hallazgos clínicos y patológicos en este primer reporte son consistentes con publicaciones previas y confirman el patrón de daño alveolar difuso asociado a agregados
de macrofagos intraalveolares y microtrombosis; ademas esteatosis macro y microvesicular hepatocitica, junto a grados variables de necrosis.
Palabras clave: SARS-COV-2; COVID-19; Patología; Inmunohistoquímica (fuente: DeCS BIREME).
The pandemic caused by the infection of the new coronavirus 2019, or as it is also known, coronavirus of Severe Acute Respiratory Syndrome 2019 (SARS-CoV-2), is causing severe damage to health and economic systems worldwide, with more than six million infected and more than three hundred and eighty thousand deaths globally to date(1). This disease appeared towards the end of 2019 in Wuhan, a commercial city in central China, in which it caused the death of more than one thousand eight hundred people and infected more than seven thousand individuals in the first fifteen days of the epidemic. The international committee on virus taxonomy named the virus SARS-CoV-2 and the disease COVID-19(1,2)
The coronavirus family (CoV) is a kind of single-stranded, encapsulated RNA virus with a very wide range of natural sources. SARS-CoV-2 is a member of the beta-coronavirus along with SARS-CoV and MERS-CoV, with whom it shares similarities in its genome, biology and clinic. These viruses cause respiratory, enteric, liver, and neurological diseases. So far, more than 37,000 SARS-CoV-2 genomes have been evaluated worldwide according to GISAID, with a large number of genomic variants being found.(3)
It has been identified that male sex, age over 65 years and smoking are risk factors for disease progression in patients with COVID-19. Underlying diseases such as hypertension, diabetes, cardiovascular and respiratory disease are higher in a statistically significant proportion in critical and fatal patients compared to non-critical patients.(4)
Multiple studies show that an inadequate systemic inflammatory response, predominantly pro-inflammatory, with the so-called cytokine storm, plays a very important role in the pathogenesis of the disease, especially in critical disease and death, associated with acute respiratory distress syndrome and viral sepsis with multiorgan failure.(5) The cytokine storm can initiate viral sepsis and inflammation-induced pulmonary injury leading to other complications including pneumonitis, acute respiratory distress syndrome (ARDS), respiratory failure, shock, multiorgan failure, and death.(6)
ARDS is a lung disease that makes it difficult for oxygen to enter the circulation, and it has been shown histopathologically to be the cause of mortality in most respiratory disorders, including fatal cases of SARS-CoV, MERS-CoV and SARS-CoV-2. More than 40 candidate genes associated with the development of ARDS to include ACE2, IL-10, tumor necrosis factor (TNF), and vascular endothelial growth factor have been found.(7) This could explain the severe disease and death of patients without apparent negative prognostic risk factors and the inadequate immune response associated with disease progression.
There are several publications that describe the clinical and epidemiological characteristics; however, the reports of the histological characteristics, as well as the demonstration of the presence of the virus in the tissue are relatively limited. The reports describing the histopathological characteristics show changes in the lung mainly corresponding to diffuse alveolar damage and microthrombosis, focal necrosis of cardiac myocytes, nonspecific degenerative changes in other organs, being similar to that observed in SARS-CoV and MERS-CoV(8-12) virus infection. Pathology is a tool that highlights the interacting pathophysiological mechanisms in tissues affected by a disease.
In this paper we will try to elucidate the characteristics of the host and the infectious agent that could have a relationship with the prognosis of patients infected with SARS-CoV-2, which includes describing the clinical and laboratory characteristics related to prognosis already defined, the presence of polymorphisms in inflammatory and epithelial genes that have a relationship with susceptibility to developing ARDS, genotyping by next-generation sequencing SARS-CoV-2 from nasopharyngeal swab or endotracheal tube aspiration, histopathological study of lung biopsies, liver and possibly kidney or other organs as well as identify the presence of SARS-CoV-2 by RT-PCR in these tissues from the tissue embedded in paraffin. An attempt will be made to define a relationship between these factors and the course of the disease divided between mild disease (outpatient), in hospitalized patients (moderate to severe disease), in intensive care (critical disease) and deceased patients.
We believe that an understanding of pathogenic mechanisms is indispensable for a rational and effective treatment of diseases. Since a large part of the population is expected to become infected with SARS-CoV-2, we consider it very important to determine what factor or factors and in what type of patients the disease will have a negative progression, and will cause severe harm to society, health system and economy, to take appropriate measures aimed at primary, secondary and tertiary prevention, including the use of appropriate medicines for rational treatment.
For this, strict biosecurity protocols for biopsy and sample management will be applied, as well as informed consent for the performance of this procedure and the taking of biopsies.
The general objective of the study is to evaluate the clinical, pathological, virological and genetic characteristics of the immune response of patients diagnosed with SARS-CoV-2 infection and to look for a relationship of these with the unfavorable course of the disease.
METHODS
Project phases
We have divided the work into three phases. In the first phase, our objectives will be to evaluate the clinical-epidemiological characteristics of patients infected with SARS-CoV-2 with all the degrees of severity of the disease, the histopathological characteristics of tru-cut biopsies of lung, liver, and other organs of deceased patients with SARS-CoV-2 infection and determine the presence of SARS-CoV-2 in tissue samples by tru-cut biopsy using RT-PCR.
In the second phase, we will seek to determine the genotype of SARS-CoV-2 in samples of nasopharyngeal swab and/or endotracheal tube aspiration by next-generation sequencing in patients infected with SARS-CoV-2 in the different degrees of disease severity.
In the third phase we will seek to determine the presence of polymorphisms or other genomic alterations in the genes related to the inflammatory response and risk of respiratory distress in patients infected with SARS-CoV-2 of the different degrees of disease severity.
In phases two and three, a relationship between the genotype of the virus and the genes of the inflammatory response and the risk of ARDS with the severity of the disease will be sought.
Type and general design of the study
The study is a descriptive work correlational, longitudinal and retrospective based on the review of clinical histories, sampling (blood and swab naso-oropharyngeal or aspiration of the endotracheal tube) and the study of laminae with histological and immunohistochemical sections in the Pathological Anatomy Department of the Hospital Nacional Carlos Seguín Escobedo (HNCASE) from lung tru-cut biopsies, liver and other organs to patients who died in the intensive care unit (ICU) and internal medicine hospitalization.
Study universe, selection and sample size
The population will be constituted by the cases diagnosed with SARS-Cov-2 infection by molecular method (RT-PCR) or rapid test in the care network Arequipa of EsSalud, with mild/moderate, severe, critical and deceased disease. There will be no sampling, we will work with the population because it is small and accessible. The medical records will be reviewed by extracting them from the clinical and hospitalization records area or ICU of the care network Arequipa, as well as from the management system. Histology sheets will be evaluated in the Pathological Anatomy Service of HNCASE. Molecular testing in blood and nasopharyngeal swab will be performed on 40 patients, who will be referred to an external molecular biology laboratory with genetic sequencing.
Study protocol
Histopathological study of biopsies:
The first phase of the study will focus on patients with COVID-19 in whom post-mortem biopsies are performed in the ICU. Clinical and laboratory characteristics will be described by reviewing medical records. The laboratory characteristics shall be drawn from the clinical records or from the management system. Tru-cut needle biopsies will be taken in the ICU immediately after the patient’s death. The samples will be submitted to the Pathological Anatomy Department of the HNCASE, where they will undergo conventional histological processing and immunohistochemical staining to define inflammatory cell populations, which include pankeratin, CD3, CD20, CD4, CD8, zimgrana B, myeloperoxidase (MPO), CD61, CD56, CD138, CD117 and CD68.
Virus identification in tissue biopsies:
In this first phase, the RT-PCR study for the identification of the viral genome in the biopsied tissues will also be carried out. To perform the RT-PCR of the tissue, the procedure begins with the classification of the sample by histopathology to define the presence of tissue parenchyma with pathological changes. Five 10µm cuts shall be made, from which the viral RNA will be removed, or, failing that, by a previous fresh evaluation of the tissue by light microscopy. The mix is prepared with defined kits covering a screening region and a definitive one. The thermocycler protocol shall then be amplified in real time and the results analysed.
Next generation sequencing for viral genotyping and the inflammatory response / risk of acute respiratory distress:
Second phase will comprehend the research through next generation sequencing of nasopharyngeal and oropharyngeal swab or aspiration of the endotracheal tube of positive patients for SARS-CoV-2 infection. Third phase will comprehend the research through next generation sequencing of peripheral blood samples of positive patients for SARS-CoV-2 infection in approximately 40 patients in order to determine genetic polymorphism of inflammatory response and predisposition to developing acute respiratory distress syndrome (ARDS) and sepsis.
Peripheral blood (2 ml) sample will be extracted into PAXgene® tubes (BD Biosciences), heparin tube (2 ml) and a nasopharyngeal swab. Five days after hospitalization, a second blood collection will be performed. DNA will be extracted from the peripheral blood samples which were collected in the PAXgene® tubes by using the AllPrep DNA/RNA Mini kit (Qiagen). Peripheral blood samples obtained from heparin tubes will be centrifuged in order to obtain plasma and will be preserved at -80. The Magmax Viral Isolation kit (Thermofisher) will be used for the extraction of SARS-CoV-2 (swab) RNA. RNA quality and concentration will be examined. The extracted RNA will be converted to cDNA by using the SuperScript™ VILO™ cDNA Synthesis kit. Genetic libraries will be prepared manually from the blood and virus cDNA. The Ion AmpliSeq RNA Inflammation Response Research inflammation panel (683 immune response genes) will be used for peripheral blood libraries. The Ion AmpliSeq SARS-CoV-2 panel, which contains 237 amplicons specific for SARS-CoV-2 (99% of the genome), will be used for different SARS-CoV-2 strain libraries. Preparation of the tempering and loading of the Ion Chip 540 will be carried out into the Ion Chef equipment. Sequencing runs will be performed on the Ion GeneStudio S5 equipment. Primary data analysis will be performed on Torrent Suite software. The expression matrix, differentially expressed genes and gene enrichment analysis will determined between patients with favorable evolution and those who developed severe COVID-19. The host immune profile will be correlated with the genetic variants of SARS-CoV-2. A predictive statistical model will be used in order to establish an inflammatory/viral profile which determines the progression into severe COVID-19. For the genes which were not included in the present kit (including ACE-2, HMOX2, ANGPT2, SOD3), RT-PCR will be used with the aforementioned methodology.
In order to perform second and third phases, clinical and laboratorial characteristics of positive patients for SARS-CoV-2 infection will be described through molecular and rapid tests, both outpatient and hospitalized through coordination with epidemiology, internal medicine and ICU areas. In non-hospitalized patients, direct questioning will be carried out, and in hospitalized and deceased patients, medical records will be reviewed. Laboratory characteristics will be extracted from medical records or from the management system.
Procedures to guarantee ethical aspects
The present research will benefit the institution and to our patients: it aims to determine which factors are related to a negative and fatal outcome of patients infected with SARS-CoV-2. Therefore, if a relationship with the genetic factor of the host of the virus, the taking of appropriate prevention and therapy may be sought. There are no similar publications in the current medical literature. In that way, it would be a very significant contribution to the study and understanding of this disease globally.
The present research does not aim to modify the diagnosis or treatment of involved patients, as well as the disease course.
The present research involves the participation of human beings and their biological samples. A total amount of approximately 40 cases will be recruited for genetic sequencing and 40 cases for taking post mortem biopsies, with no age range determined. There will be no type of patients discrimination.
Informed consent forms will be used for alive patients and for family members of the patients that are entering to hospitalization. The informed consent for patients who are going to undergo the viral genetic sequencing study and inflammatory genome will be performed directly to the patient in case it is possible, since the study will be taken to patients with mild to critical illness. In case the patient is not able to give the form (critical condition or dead), the family members will do. In case the consent is obtained from family members, after the patient passes away, the order established by the 13rd Article of Peruvian Civil Code will be considered in order to decide in terms of a necropsy, cremation and sepulture, that means, first, the spouse; then, the descendants; and lastly, ascendants or siblings.
Payment will not be made to study participants. A final report will be made to the patients or relatives of their results if this is authorized by the patients or their relatives.
The realization of this work will not lead any harm to those involved, both health personnel and patients, since it will be performed under defined management protocols, or to third parties.
The results of this study will seek to be published in an indexed medical journal, as well as be delivered to the respective EsSalud authorities as requested.
The present research work will maintain confidentiality of names and other personal identification resources of patients by giving them a study individual code. The identification of the patients through names and last names will be collected, they will be immediately codified, and they will be treated in accordance with this codification. The list with personal identification and its codification will by only managed by the main researcher. The secondary researchers will only manage the codified list, complying with the personal data protection law.
Since the samples for genetic study will be taken to an external laboratory, it its established that the samples and genetic information will not be stored nor will additional information will be extracted for the purposes of the present study.
The present research has the approval of the Specific Research Ethics Committee for Institutional COVID-19.
RESULTS
Clinical and pathologic characteristics of initial cases are resumed on Table 1.
Situational status of COVID-19 in EsSalud Arequipa network
For the time being (June 4) at Arequipa Region, 4 067 positive cases and 85 deaths have been registered due to this disease. At EsSalud Arequipa Healthcare Network, the HBCASE has become into the reference hospital for COVID-19 in social security. At this hospital, we have 158 hospitalization beds, and 23 ICU beds for COVID-19 patients. The amount of hospitalized patients between April to May 2020 was 136, and for the time being, 55 deaths have been registered at HBCASE due to the abovementioned cause. Of the 136 hospitalized cases, 64% correspond to men and 40% to women. Regarding the distribution by age group, 58% correspond to people over 60 years of age.
Clinical description of the first cases evaluated
For the time being, we have included in our research seven deceased patients in the ICU who have undergone tru-cut needle biopsied. Most of them were male (n°: 4, 57%), the average age was 75 years, 42% of the patients were overweight, 42% had at least one comorbidity, which in order of frequency were: high blood pressure (28%), chronic kidney disease (28%), and diffuse interstitial lung disease (14%). Regarding the clinical picture of admission, the time of illness before admission was 6.8 days on average. Most frequent symptom was dyspnea. Cough and feeling of thermal rise were additional symptoms in frequency. As for the blood count, the majority presented leukocytosis with neutrophilia and lymphopenia in their evolution. The relationship neutrophil-lymphocyte was markedly elevated. Patients developed severe disease and entered ICU on average 8.1 days after the onset of the disease. Regarding the severity scales, the average APACHE II Score of the patients was 13.4 points; SOFA Scores at admission, third and seventh day were 6, 8 and 7 respectively. All patients underwent mechanical ventilation, the average ratio between pao2 to fio2 (pao2/fio2) on the first, third and seventh day was 136, 171 and 190, respectively. The severity distribution of acute respiratory distress syndrome, using the Berlin definition, uncorrected for height, was moderate in most cases (85%). The organic failures developed by the patients were, in order of frequency: acute kidney failure in 85%, acute liver failure in 42%, and acute heart failure in 14% of cases. Two positive bronchial secretion cultures were obtained, one with resistant multidrug A. baumanii (MDR) and the other with P. aeruginosa MDR, and additionally a positive blood culture for A. baumanii in a third patient. The average days of stay in the ICU of the patients was eleven days.
Pathological findings of the first patients
Lung biopsies showed predominantly intra-alveolar macrophage hyperplasia with accumulation within the alveolar space, which ranged from mild to severe. In addition, mild to moderate hyperplasia of intra-alveolar pneumocytes is observed with large cells and binucleated and atypical forms, with eosinophilic nuclei (similar to virocytes) in some cases. Several cases presented deposits of intra-alveolar fibrinoid material in aggregates with formation of hyaline membranes, which were focal. Additionally, a mild to moderate inflammatory infiltrate composed predominantly of histiocytes/monocytes and neutrophils is observed in the interalveolar septa, confirmed with immunohistochemical staining for CD68, CD4, and myeloperoxidase. With respect to the lymphoid infiltrate, in all cases it was very mild, composed of predominantly CD8-positive T cells, and in two that showed severe inflammation, CD4 and CD8 T lymphocytes were evident. CD20-positive B cells, CD138-positive plasma cells, natural CD56-positive killer cells, CD117-positive mast cells were almost not evident. In the vast majority of cases, intravascular fibrin deposits associated with the accumulation of inflammatory cells composed of neutrophils and monocytes, representing microthrombosis, were observed.
Liver biopsies showed predominantly macrovesicular steatosis and two cases showed microvesicular steatosis. In addition, varying degrees of necrosis and mild portal and lobular inflammation were observed.
Table 1. Pathological and clinical characteristics of initial cases
| N° | Age | Sex | BMI | Comorbidities | Time from onset of symptoms to development of ARDS | Hematological abnormalities | Relation neutrophils: lymphocytes start and end | Severity ARDS | Acute failure of target organ | APACHE II Score | SOFA Score | Treatment received (days) | Main pulmonary pathologic findings | Main hepatic pathologic findings |
| 1 | 71 | M | Overweight | No | 8 | Leukocytosis with neutrophilia and lymphopenia | 17-6,8 | Severe | Renal and hepatic | 15 | 11,5 | HCQ, L/R,C (3) | Hyaline membranes. hyperplasia and detachment of pneumocytes, hyperplasia of alveolar macrophages, microthrombosis, multinucleated cells. | Macrovesicular steatosis and focal necrosis of hepatocytes. |
| 2 | 83 | M | Normal | AHT, CKD | 7 | Leukocytosis with neutrophilia and lymphopenia | 22-47 | Moderate | Renal, hepatic and cardiac | 14 | 8 | HCQ, L/R,C (2,2,3) | Hyaline membranes, hyperplasia and detachment of pneumocytes, microthrombosis, multinucleated cells, fibrin and intra-alveolar hemorrhage. | Microvesicular steatosis, diffuse necrosis. |
| 3 | 76 | M | Normal | No | 8 | Leukocytosis with neutrophilia and lymphopenia | 30-32 | Moderate | Renal | 16 | 6,5 | L/R (4) | Hyperplasia and detachment of pneumocytes, hyperplasia of alveolar macrophages, microthrombosis, multinucleated cells, fibrin y intra-alveolar hemorrhage. | Microvesicular steatosis |
| 4 | 71 | M | Overweight | CKD, Diffuse ILD | 8 | Leukocytosis with neutrophilia and lymphopenia | 10-12 | Moderate | Renal and hepatic | 15 | 7,5 | No | Microvesicular steatosis | |
| 5 | 79 | F | Overweight | No | 6 | Mild anemia, leukocytosis with neutrophilia and lymphopenia | 6-11 | Moderate | Renal, hepatic and cardiac | 11 | 4,3 | HCQ, L/R,C (1,1,3) | Hyaline membranes, fibrin, hyperplasia and detachment of pneumocytes. | Microvesicular steatosis |
| 6 | 67 | F | Normal | No | 14 | Mild anemia, leukocytosis with neutrophilia and lymphopenia | 12-Feb | Moderate | Renal | 12 | 8,3 | C (3) | Hyperplasia and detachment of pneumocytes, hyperplasia of alveolar macrophages, microthrombosis, multinucleated cells, fibrin y intra-alveolar hemorrhage. | Microvesicular steatosis |
| 7 | 80 | F | Normal | HTA | 4 | Lymphopenia | 7,4-7,7 | Moderate | No | 11 | 6,6 | No | Hyperplasia and detachment of pneumocytes, hyperplasia of alveolar macrophages, microthrombosis, multinucleated cells, fibrin and intra-alveolar hemorrhage |
AHT: Arterial hypertension, CKD: Chronic kidney disease, Diffuse ILD: Diffuse interstitial lung disease, HCQ: Hydroxychloroquine, L/R: Lopinavir/ritonavir, C: Corticosteroids
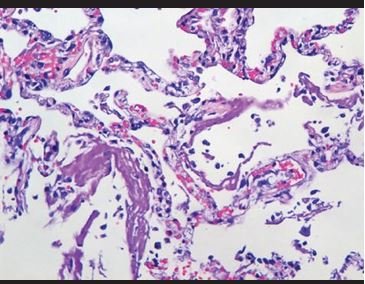 Figure 1. Intra-alveolar hyaline material deposits and vascular congestion, forming hyaline membranes. |
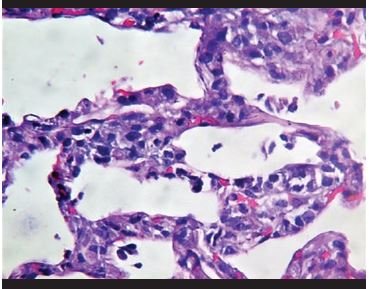 Figure 2. Inflammatory infiltrates of neutrophils and histiocytes on interalveolar septa. |
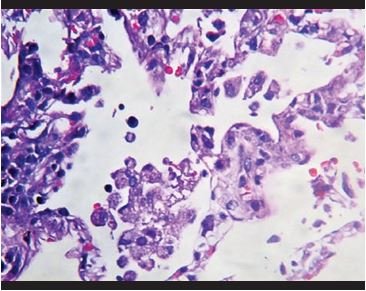 Figure 3. Hyperplasia of intra-alveolar macrophages. |
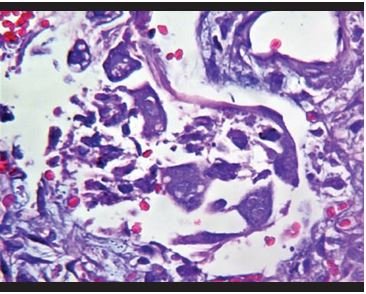 Figure 4. Binucleated epithelial cells with prominent eosinophilic nucleoli, similar to virocytes. |
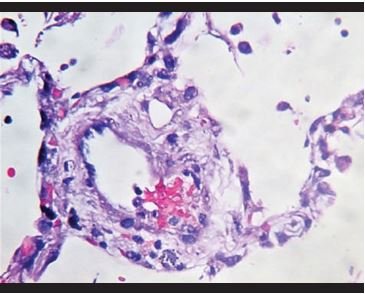 Figure 5. Vascular congestion with fibrin deposits and adherence of inflammatory cells to the vascular wall. |
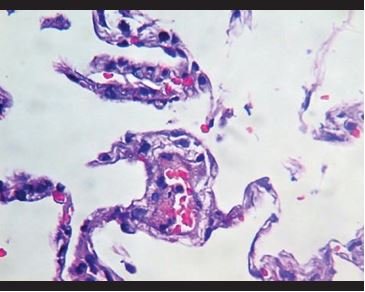 Figure 6. Vascular congestion with fibrin deposits and adherence of inflammatory cells to the vascular wall. |
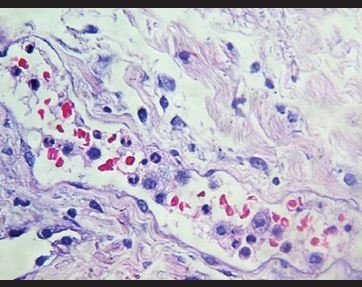 Figure 7. Congestion with intravascular neutrophils and monocytes. |
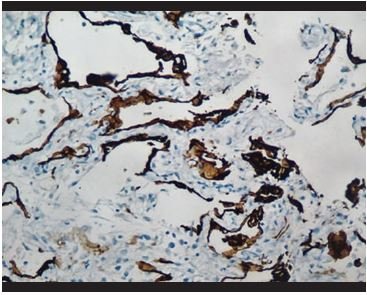 Figure 8. Immunohistochemical staining for pankeratin (AE1/AE3). Hyperplasia and detachment of alveolar pneumocytes is evidenced. |
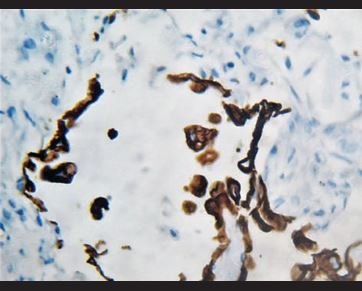 Figure 9. Immunohistochemical staining for pankeratin in which it is evidenced that atypical cells are positive, corresponding to epithelial cells line. |
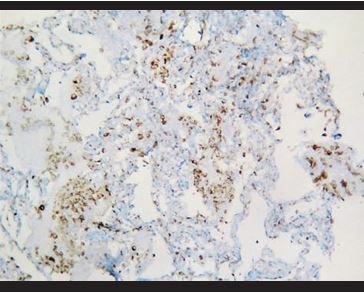 Figure 10. Immunohistochemical staining for CD68, in which it is evidenced the presence of aggregated intra-alveolar macrophages. |
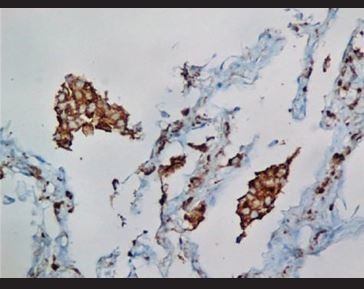 Figure 11. Immunohistochemical staining for CD68, in which it is evidenced the presence of aggregated intra-alveolar macrophages in a desquamative pneumonitis pattern. |
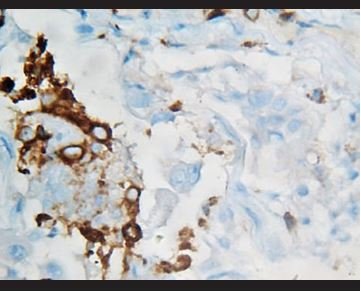 Figure 12. Immunohistochemical staining for CD68, in which there are evidenced positive intra-alveolar macrophages, while atypical binucleated cells are negative. |
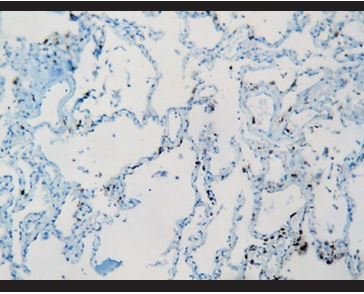 Figure 13. Immunohistochemical staining for CD3: Few interstitial T lymphocytes. |
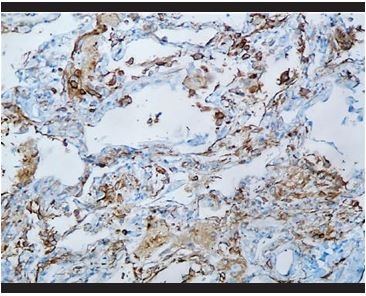 Figure 14. Immunohistochemical staining for CD4: Presence of hyperplasia of intra-alveolar and interstitial histiocytes. |
 Figure 15. Immunohistochemical staining for Mieloperoxidase: Presence of neutrophils in the peri-alveolar interstitial tissue. |
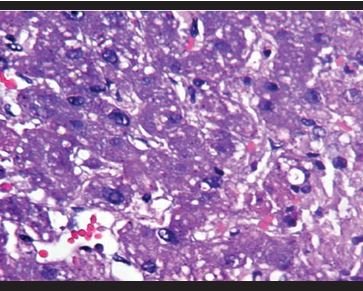 Figure 16.Liver: Microvesicular steatosis. |

Figure 17. Liver: Macrovesicular steatosis with hepatocytic balonizing degeneration and focal necrosis.
The initial seven deceased patients were predominantly male, older middle age, showed overweight, and comorbidities such as hypertension, chronic renal disease, and diffuse interstitial lung disease. Additionally, the blood count showed leukocytosis with neutrophilia and lymphopenia in most patients, with a high neutrophil: lymphocytes in all patients. In a relevant way, all had acute renal failure at the end, and lower cardiac grade. Lung biopsies confirm the involvement reported in previous studies corresponding to the pattern of diffuse alveolar damage with epithelial and endothelial injury, with the addition that in our cases the presence of an important macrophageal inflammatory component with intra-alveolar aggregates was very prevalent; the presence of neutrophils in the alveolar walls and in the interstitium was also notorious. The lymphocyte component was minimal and represented by positive CD4 and CD8 T cells. The presence of microthrombosis in small-caliber vessels was also evident. Liver biopsies showed macro and microvesicular steatosis, along with varying degrees of necrosis
CONCLUSION
These cases represent the first step in the study. Clinical and pathological findings are consistent with previous publications and confirm the pattern of diffuse alveolar damage. We hope to complete it with a higher number of cases and the rest of the molecular biology protocol, which we believe will provide important knowledge on the pathophysiology of this disease.
Authorship contributions: The authors participate in the genesis of the idea, project design, data collection and interpretation, analysis of results and preparation of the manuscript of this research work.
Financing: The financing process for molecular studies is in process.
Conflict of interest: The authors declare that they have no conflict of interest.
Received: June 3, 2020
Approved: June 10, 2020
Correspondence: Mauricio Postigo Mac Dowall
Address: Pathology Department, Carlos Seguín Escobedo-EsSalud hospital. Esq. Peral y FIltro s / n Arequipa, Arequipa-Peru.
Telephone: 958348121
Email: maupostigomd80@gmail.com
BIBLIOGRAPHIC REFERENCES
