ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2021 - Universidad Ricardo Palma10.25176/RFMH.v21i4.4023
STANDARDIZATION AND VALIDATION OF A WESTERN BLOT FOR THE DIAGNOSIS OF HUMAN IMMUNODEFICIENCY VIRUS
ESTANDARIZACIÓN Y VALIDACIÓN DE UN WESTERN BLOT PARA EL DIAGNÓSTICO DEL VIRUS DE INMUNODEFICIENCIA HUMANA
Eduardo Miranda-Ulloa1,a, Soledad Romero-Ruiz1,b, Bernardina Amorín-Uscata2,c, Kevin Serrano-Segura1,b, Ronal Briceño-Espinoza1,d, Fany Cárdenas-Bustamante1,b
1 Laboratorio de Referencia Nacional Virus de Transmisión Sexual VIH/SIDA, Centro Nacional de
Salud Pública, Instituto Nacional de Salud. Lima, Perú.
2 Instituto de Medicina Tropical de São Paulo, São Paulo, Brasil.
a Biólogo, magíster en Microbiología
b Biólogo
c Biólogo magíster en Ciencias
d Tecnólogo médico.
ABSTRACT
Objectives: To standardize and validate a western blot test for the diagnosis of human immunodeficiency virus. Methods: A prospective observational study was carried out during 2017 and 2018. The western blot test was standardized, using the polyacrylamide gel electrophoresis technique with sodium dodecyl sulfate (SDS PAGE), being the nitrocellulose blot strips prepared with an Optimal HIV-1 antigen concentration of 2.71 µg / mm. The western blot was validated in the laboratory against 400 reference samples (300 sera and 100 plasmas): 200 positive and 200 negatives for antibodies against HIV-1, being the reference test the Immunoblot of the Fujirebio brand. Diagnostic performance parameters were estimated using Epidat v3.1 and Excel. Results: Eight important bands of the HIV-1 antigen were identified: p17, p24, p31, p39, gp41, p55, p66, and gp120. According to the Consortium for the normalization of serology for retroviruse, those that were taken as specific diagnostic bands were: p24, p31, gp41, and gp120. The sensitivity, specificity, positive and negative predictive value and validity index against sera were: 96.7%, 96.0%, 96.0%, 96.6%, 96.3%; and against plasmas: 98.0%, 100.0%, 100.0%, 98.0%, 99.0% respectively. No false positives and negatives were found, but some were undetermined. Conclusion: The development of this western blot test with proprietary technology presented similar diagnostic performance to the reference test, without showing cross-reactions, being useful for confirming HIV.
Keywords: Western Blotting; HIV; Diagnosis; Sensitivity and Specificity (Source: MeSH NLM).
RESUMEN
Objetivos: Estandarizar y validar una prueba de western blot para el diagnóstico del virus de inmunodeficiencia humana. Métodos: Se realizó un estudio observacional prospectivo durante el 2017 y 2018. Se estandarizó la prueba de western blot, usando la técnica de electroforesis en gel de poliacrilamida con dodecil sulfato de sodio (SDS PAGE), siendo las tiras blot de nitrocelulosa preparadas con una concentración óptima de antígeno de VIH-1 de 2,71 µg/mm. Se validó el western blot en laboratorio, frente a 400 muestras referentes (300 sueros y 100 plasmas): 200 positivas y 200 negativas a anticuerpos contra VIH-1, siendo la prueba de referencia el Inmunoblot de la marca Fujirebio. Se estimaron los parámetros de rendimiento diagnóstico usando el programa Epidat v3.1 y Excel. Resultados: Se logró identificar ocho bandas importantes del antígeno de VIH-1: p17, p24, p31, p39, gp41, p55, p66 y gp120. De ellas, las que se tomaron como bandas diagnósticas específicas según el Consorcio de normalización de serología para los retrovirus, fueron: p24, p31, gp41 y gp120. La sensibilidad, especificidad, valor predictivo positivo y negativo e índice de validez frente a sueros fueron: 96,7%, 96,0%, 96,0%, 96,6%, 96,3%; y frente a plasmas: 98,0%, 100,0%, 100,0%, 98,0%, 99,0% respectivamente. No se encontraron falsos positivos y negativos, pero si algunos indeterminados. Conclusión: El desarrollo de esta prueba western blot con tecnología propia, presentó similar rendimiento diagnóstico a la prueba de referencia, sin mostrar reacciones cruzadas; siendo útil para la confirmación del VIH.
Palabras Clave: Western blot; VIH; Diagnóstico; Sensibilidad y Especificidad (Fuente: DeCS BIREME).
INTRODUCTION
In Peru, within the strategies for the approach to infection by the human immunodeficiency virus
(HIV) is the coverage of the diagnosis (1), so much so that there are
serological screening tests (ELISA, chemiluminescence and rapid tests) and confirmation: indirect
immunofluorescence (IIF) and immunoblot (IB) or western blot (WB) (2,3). The IIF is a test with its
technology (4), relatively inexpensive and constitutes 95% of the
confirmations at the national level, however when the result is indeterminate or nonspecific, the IB or
WB is used for having superior diagnostic performance (3).
The commercial IB of the Fujirebio brand INNO-LIA HIV I / II Score (Online Immunoassay:
sensitivity 100.0% and specificity 96.7%) is the kit most used as a second opinion test for samples that
did not resolve IIF (5), being at the same time applied in numerous studies
as a gold standard (6-10), but its main disadvantage is that its costs are
high, approximately
$800 for 20 determinations (5).
Trademarks available from WB include MP Diagnostics HIV BLOT 2.2 (sensitivity: 100.0%;
specificity: 91.9%) (11), Biorad NEW LAV-BLOT I (sensitivity: 87.0%;
specificity: 99 , 5%) (12) and Biokit bioblot HIV-1 Plus (sensitivity:
100.0%; specificity: 91.9%) (13). Although these three brands do not report
false positive and negative results (only indeterminate), their use is very limited due to the expensive
prices attributed to their kits (1,000 to 1,200 dollars for 18 determinations) (11-13).
The western blot, immunoblot, or immunoblotting is a useful test in identifying antibodies
against HIV. It consists of separating the viral antigens by electrophoresis in polyacrylamide gels,
then transferred to a nitrocellulose membrane, which will be subsequently exposed with the antibodies of
the serum or problem plasma. When they come into contact with an anti-immunoglobulin labeled with an
enzyme, they will react immunologically, giving rise to a pattern of bands, which will be interpreted
under some of the criteria described by international organizations (14).
It should be said that, in Peru, around 1,200 annual samples are resolved by immunoblot/western
blot (15), whose expenses reach more than 60,000 dollars per year. Therefore,
due to the need for a low-cost western blot with our technology and the high prices of commercial kits,
we set ourselves the following objective: Standardize and validate a western blot to diagnose human
immunodeficiency virus.
METHODS
Design and study area
Prospective observational study of standardization and validation of a diagnostic test carried out during 2017 and 2018 at the National Institute of Health (INS) of Peru. Under this approach, the design is appropriate to estimate the capacity of a measure that allows discriminating between people with the disease and without the disease.
Population and sample
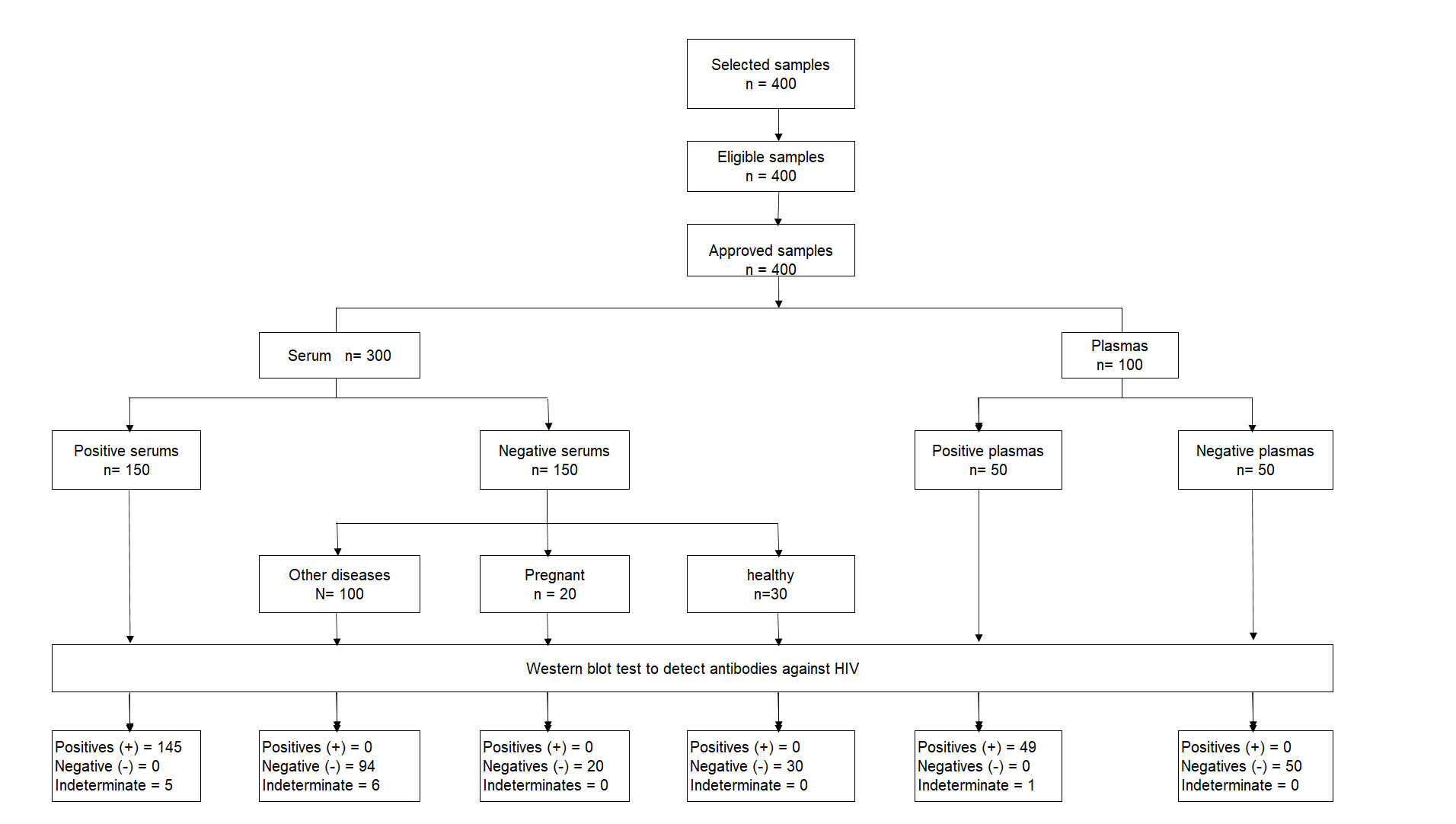
The population consisted of sera and plasmas that belonged to the serum and plasma library of the National Reference Laboratory of STV-HIV / AIDS of the INS during the years 2016 and 2017. The estimation of the sample size was defined by convenience, using a non-probabilistic sampling, with 400 reference samples: 150 HIV-1 positive sera; 150 HIV-1 negative sera (30 from healthy individuals and 120 from individuals with the following conditions: twenty with syphilis, twenty from pregnant women, twenty with rheumatoid disease (RD), twenty with hepatitis B, twenty with HTLV-1, ten with cytomegalovirus and ten with dengue); 50 HIV-1 positive and 50 negative plasmas. The Reference or Gold Standard test was the commercial IB (INNO-LIA ™ * HIV I / II Score; Fujirebio, Belgium) (5).
Variables and instruments
The standardization and validation of a diagnostic test implied considering the following
variables: Sensitivity, Specificity, Validity Index, Positive Predictive Value (PPV), Negative
Predictive Value (NPV), Youden Index, and Negative Likelihood Ratio. Three stages were previously
carried out to measure these variables.
Optimization in the preparation of the HIV-1 antigen. - The cell cultures of the H9 / HTLV-IIIB
line infected with HIV-1 were prepared, following the methodology described by Romero -Ruiz et al.
(16). The cell supernatant was washed and centrifuged three times with 0.9%
physiological saline solution (SSF) at 4,400 rpm for 15 minutes at 2 to 8 ° C. The sediment or pellet
was then resuspended with SSF. A sonicator was used to carry out the viral and cell lysis at 6 periods
of 60 decibels with 2 minutes per period and one minute of rest between periods, maintaining the cold
chain (2 to 8 ° C).
The sonicated product was centrifuged at 1,500 rpm for 10 minutes, and its supernatant was
registered as HIV-1 antigen. Finally, the Bradford method (17) was used to
quantify the HIV-1 antigen proteins.
Standardization in the preparation of Western blot strips.- This step was standardized following
electrophoresis methodology in polyacrylamide gels with sodium dodecyl sulfate (SDS-PAGE) (18). The Mini-Protean vertical electrophoresis chamber (Bio-rad) was used for
1.0 mm gels (gel size: 8.3 by 7.3 cm). The percentage of acrylamide was optimized to prepare the
resolving gel, establishing two concentrations (mixed) 9% (3.8 cm) and 15% (2 cm). The antigen
treatment, separation, and electrophoretic transfer were carried out following the methodology described
by Miranda et al. (19). Finally, the optimal concentration of the HIV-1
antigen was established to prepare the WB strips, being 2.71 µg / mm.
Optimization in the Immunoenzymatic reaction of the Western blot test.- The optimum volume that
was established in all the steps of this stage was 1 mL. The blot strips were incubated for three hours
in PBS / tween-milk (PBS / 0.1M NaCl, 0.05 M Na2 PO4, pH 7.2; Tween 20 / 0.3% tween 20; milk / 5% milk)
containing the sera or plasmas of the study at a 1/40 dilution. Next, the nitrocellulose strips were
washed five times with PBS / Tween-20 and incubated for one hour in PBS / tween-milk containing the
conjugate Anti-human IgG linked to a peroxidase, at a dilution of 1/1000. The strips were again washed
three times with PBS / Tween-20 and twice with PBS. Bands were developed against a substrate solution
(30% hydrogen peroxide at 1uL / mL and diaminobenzidine at 0.5 mg / mL in PBS pH 7.2). The reaction was
stopped by washing the strips with distilled water.
Procedures
Reading, interpretation, and validation of the Western blot test.- For the positivity criterion, the Consortium guidelines for the normalization of retrovirus serologies (CRSS) (11-14, 20) were considered. POSITIVE: A band of p24 or p31 and an ENV band (gp41 or gp120). NEGATIVE: No specific viral band present. UNDETERMINED: Any specific viral band present, but the pattern does not meet the criteria for positive. With these criteria, the WB was validated, performing the readings and interpretations of the 400 reference samples incorporated into the study.
Statistical analysis
The analysis was estimated using a contingency table, the Epidat v3.1 program, and Excel. The percentages of Sensitivity, Specificity, Validity Index, Positive Predictive Value (PPV), Negative Predictive Value (NPV), Youden Index, and Negative Likelihood Ratio were reported, considering a 95% confidence level (95% CI).
Ethical considerations
The Institutional Research Ethics Committee approved the study protocol of the National Institute of Health of Peru with the code: OI-022-14 and with Directorial Resolution N °: 418-2014-DG-OGITT-OPE / INS. Likewise, the General Office for Research and Technology Transfer approved the final report of this study with MEMORANDUM N ° 027-2019-OGITT / INS.
RESULTS
Among our findings, we were able to show eight important bands corresponding to the HIV-1 viral proteins: p17, p24, p31, p39, gp41, p55, p66, and gp120. Of these, those that were taken as specific diagnostic bands according to the CRSS were: p24, p31, gp41, and gp120 (Figure 1).
Among other findings, we highlight that against the serum samples, there were eleven indeterminate results (5 from the positive panel and 6 from the negative panel), and against the plasma samples, there was an indeterminate from the positive panel. Said results of the WB test are shown in the flow diagram of samples incorporated into the study (Figure 2).
An important highlight of this WB test is that it did not show false positives and negatives. However, only some serum samples had indeterminate results: syphilis (2/20), hepatitis B (2/20), ER (1/20), and HTLV-1 (1/20) (Table 1).
Table 1. Western blot test validation for detection of antibodies against HIV using positive, negative and interfering samples.
| Western blot VIH | ||||||
|---|---|---|---|---|---|---|
| Condition | Amount | True positives | False positives | True negatives | False negatives | Indeterminate |
| Serums | ||||||
| VIH-1 | 150 | 145 | - | 0 | 0 | 5 |
| Syphilis | 20 | - | 0 | 18 | - | 2 |
| Pregnant | 20 | - | 0 | 20 | - | 0 |
| RE* | 20 | - | 0 | 19 | - | 1 |
| Hepatitis B | 20 | - | 0 | 18 | - | 2 |
| HTLV-1 | 20 | - | 0 | 19 | - | 1 |
| CMV** | 10 | - | 0 | 10 | - | 0 |
| Dengue | 10 | - | 0 | 10 | - | 0 |
| Healthy | 30 | - | 0 | 30 | - | 0 |
| Plasmas | ||||||
| VIH-1 | 50 | 49 | - | 0 | 0 | 1 |
| Negative to VIH-1 | 50 | - | 0 | 50 | - | 0 |
| Total | 400 | 194 | 0 | 194 | - | 12 |
Among the main achievements, we show that the results of the validation parameters (sensitivity, specificity, PPV, NPV and Validity Index) for our WB test were higher than 96.0%. In the same way, the Youden index with the values of 0.93 and 0.98, confirms a minimal possibility of obtaining false positives or false negatives; as well as a low negative likelihood ratio of 0.03 and 0.02, which is consistent with the other results (Table 2).
Table 2. Western blot test parameters for the detection of antibodies against HIV against serum and plasma samples
| Western blot VIH | ||
|---|---|---|
| Parameters | Serum | Plasma |
| IC Value(95%) | IC Value(95%) | |
| Sensitivity(%) | 96,7 (93,5 - 99,9) | 98,0 (93,1-100,0) |
| Specificity (%) | 96,0 (92,5 - 99,5) | 100,0 (99,0 - 100,0) |
| Validity index (%) | 96,3 (94,0 - 98,6) | 99,0 (96,6 - 100,0) |
| Positive predictive value (%) | 96,0 (92,6 - 99,5) | 100,0 (99,0 - 100,0) |
| Negative predictive value (%) | 96,6 (93,4 - 99,9) | 98,0 (93,3 - 100,0) |
| Youden index | 0,93 (0,88 - 0,97) | 0,98 (0,94 - 1,02) |
| Negative likelihooh ratio | 0,03 (0,01 - 0,08) | 0,02 (0,0 - 0,14) |
DISCUSSION
The reference test used in the present study is the most used in Peru (2,3,6,15), and other
countries (5,7-10), the insert reports a sensitivity of
100%
and specificities of: 96.7%
(blood bank)
and 96.1% (clinical samples). This kit incorporates recombinant proteins and synthetic peptides from
HIV-1 and HIV-2 (5). The sensitivity and specificity of our WB using antigenic
proteins from HIV-1 lysis were higher than 96.0%; even so, we did not have false negatives and
positives, which allows us to deduce that our results for the same parameters are similar.
Western blot kits NEW LAV BLOT I (Biorad) (12) and bioblot HIV-1 plus
(Biokit) (13) using the CRSS positivity criteria, report sensitivities of
99.5% and 94.9% and specificities 87.0% and 91.9%, respectively. At the same time, they report that they
did not have false negatives and positives, only indeterminate (12,13). Likewise, the Immunoblot Recom Line HIV-1 & HIV-2 IgG kit (Mikrogen
diagnostik) (21), reports a sensitivity of 100% and specificities of: 99.3%
(blood bank), 98.5% (samples clinical) and 96.4% (interfering). Our WB test for the same parameters
showed results comparable to the three western blot / Immunoblot brands described.
Due to the indeterminate results obtained, a sensitivity of 100.0% was not achieved; This could
be due to the fact that the antibodies against p24 and p31 decrease during the course in the AIDS phase,
which causes a displacement of the interpretation from positive to indeterminate (11); however, we do not know if the samples came from patients in this phase.
To establish specificity (sera), indeterminate results represented 4.0% (6/150); It should be
said that, for the calculations in other validation studies, indeterminates are not considered in the
category of false positives (22), being this way, we would have a global
specificity of 100.0%, in the 144 samples (144/144). In general, the indeterminate (12/400) that we
obtained (Table 1) does not affect the diagnostic efficiency of our WB since the
Peruvian technical
standard indicates to perform the HIV-1 viral RNA PCR test (viral load) on the indeterminate or
proviral-HIV-1 DNA PCR (2).
Our standardized and validated test followed a WB design developed for parasites, which had
excellent results in Peru (19,23-25). The different
commercial
brands of immunoblot/western blot for
HIV use a conjugate containing the enzyme alkaline phosphatase, the substrate 5-Bromo-4-chloro-3-indolyl
phosphate (BCIP) and nitro blue tetrazolium (5,11-13,21). While in our WB we used a conjugate
containing peroxidase, the substrate hydrogen peroxide and diaminobenzidine as a chromogen, similar to
these reagents they were used in the WB DAVIH-BLOT kit from Cuba, which showed good diagnostic
performance against samples serum, urine and oral fluid (26).
In addition, the strip size format and the volume to be used are similar to the Reference test
(5), while the other commercial brands use larger blot strips and consequently
require double the volume of reagents. The aforementioned conditions are important to highlight because
our methodology saves, since it uses fewer reagents. At the same time, we prepare our viral antigen,
having the advantage that the INS has a Biosafety Level III Laboratory, where it is performing the
maintenance and development of cell cultures of the H9 / HTLV-IIIB line infected with HIV-1.
Consequently, our WB test would cost up to ten times less than commercial kits.
The WB test was standardized and validated in the laboratory, being a limitation of the study in
completing its validation in the field. It can be implemented in the reference laboratories of Peru.
CONCLUSION
The results of the parameters obtained in the WB test for the detection of antibodies against HIV qualify it as a test of good diagnostic performance and make it useful for serological confirmation. Consequently, we recommend its use in the National Reference Laboratory of the INS Sexually Transmitted Virus Test as an alternative assay to commercial confirmatory tests in addition to significantly reducing costs in HIV confirmation.
Authorship contributions: The authors participated in the conception and design of the
project; collection, analysis and interpretation of the results; writing, critical review and
approval of the final version of the article.
Funding sources: The project was financed by the competitive fund of the Centro Nacional
de Salud Pública del Instituto Nacional de Salud de Perú
Declaration of conflicts of interest: The authors declare that they have no conflicts of
interest in the publication of this article.
Received: June 24, 2021
Approved: July 14, 2021
Correspondence: Eduardo Fernando Miranda Ulloa
Address: Defensores del Morro 2268, Chorrillos – Lima, Perú.
Telephone: (051) 977783088
E-mail: fernandoul@hotmail.com ; emiranda@ins.gob.pe
REFERENCES