ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i4.4616
CRITICAL VALUES FOR AUTOMATED BLOOD COUNTS AND PERIPHERAL BLOOD SLIDES
VALORES CRÍTICOS PARA HEMOGRAMAS AUTOMATIZADOS Y FROTIS DE SANGRE PERIFÉRICA
Huerto JL1,a, Villaorduña AM1,a
1Hematology, Hemotherapy and Blood Bank Service, Hospital Nacional Alberto Sabogal Sologuren, Callao, Peru
aSurgeon, specialty in clinical pathology.
ABSTRACT
Objective:The reporting of critical values is considered a necessary practice in clinical pathology laboratories since immediate communication determines therapeutic decisions that can save lives. The choice of critical values and the way they are reported must be a joint decision between laboratory specialists and clinical professionals from each health facility since they must be integrated into the dynamics of the services involved. Among the critical values that have been chosen for this review, we have included quantitative parameters, whose processing is carried out in automated hematology analyzers, and morphological findings in peripheral blood smears, which trained professionals evaluate.
Keywords:Pathology; Blood; Cytology; Laboratories; Microscopy (fuente: MeSH NLM).
RESUMEN
Objective:El reporte de valores críticos se considera una práctica necesaria en los laboratorios de patología clínica, pues su comunicación inmediata determina decisiones terapéuticas que pueden salvar vidas. La elección de los valores críticos y el modo en que son reportados debe ser una decisión conjunta entre los especialistas del laboratorio y los profesionales clínicos de cada establecimiento de salud, ya que deben integrarse a la dinámica de los servicios involucrados. Entre los valores críticos que se han escogido para esta revisión hemos incluido parámetros cuantitativos, cuyo procesamiento se ejecuta en analizadores hematológicos automatizados, y hallazgos morfológicos en los frotis de sangre periférica, lo cuales son evaluados por profesionales capacitados.
Palabras Clave: Patología; Sangre; Citología; Laboratorios; Microscopía. (fuente: DeCS BIREME).
INTRODUCTION
Critical values in clinical pathology are defined as results that reflect imminent threats to patients' lives unless therapeutic measures are administered promptly (1). These results are often far outside the upper or lower limits of the “normal” ranges. Therefore, one should not fall into the error of considering "critical" any result that exceeds the reference values, that is, the expected values in healthy individuals (1). The crucial difference between a critical and abnormal value is that, in the first case, the result indicates an urgent danger to the patient's life.
Likewise, a clear distinction must be made between critical and vital values. The latter are “values that represent a pathophysiological state so different from normal as to be life-threatening and for which corrective measures can be taken, but for which prompt action is not so crucial (2).” As we can see, a vital value is as important as a critical value, but the immediacy of its report is not so decisive.
Currently, clinical laboratories are responsible for determining and communicating their critical values, defined according to the complexity of each service, the type of users it serves, the resources available, and the local epidemiological profile. The effective practice of reporting critical values implies a decrease in mortality and morbidity in health systems and a reduction in costs derived from preventable complications and damages (3).
In hematology laboratories, there is a lack of consensus about which results should be considered critical, as well as discrepancies in terminology and reporting mechanisms (4). Although selecting critical values is the responsibility of each clinical pathology service, we consider it pertinent to propose a list of results (explaining, for each one, the reasons that justify their selection) that serves as a starting point for other laboratories to define or refine their results. own critical value reporting processes.
CRITICAL VALUES FOR AUTOMATED BLOOD CELL COUNTS
Hemoglobin less than 7 g/dL
The mortality of postoperative patients who refused packed red cell transfusions for religious reasons has been investigated. These studies have concluded that hemoglobin levels between 7 g/dL and 8 g/dL are associated with a low risk of mortality, while levels below 6 g/dL are associated with extremely high levels of mortality (5- 7). Bleeding (38.5%), respiratory failure (35.9%), kidney failure (28.2%), sepsis (20.5%), and cardiac infarction (12.8%) were the main causes of death for these patients (6).
Likewise, the AABB's most recent clinical practice guideline (American Association of Blood Banks) recommends a hemoglobin threshold of 7 g/dL to indicate transfusion of packed red blood cells in hemodynamically stable patients (8).
Hemoglobin greater than 22 g/dL and hematocrit greater than 65% in neonates
A hematocrit greater than 65% or a hemoglobin level greater than 22 g/dL define neonatal polycythemia: an abnormal elevation in the mass of circulating erythrocytes that generates hyperviscosity blood (9-11). Hyperviscosity in neonates is associated with decreased blood supply to organs such as the brain, heart, lungs, and intestine. The resulting hypoperfusion can lead to life-threatening conditions such as systemic hypoxia, hypoglycemia, necrotizing enterocolitis, seizures, and other neurological symptoms (10). Although the optimal management for neonates with polycythemia has not been standardized, exchange transfusion is usually indicated; however, this decision remains controversial (10-11).
Hematocrit greater than 65% in
heart patients In patients with cyanotic congenital heart disease, the main hematological complication consists of an increase in hematocrit and hemoglobin concentration. These changes are secondary to the production of erythropoietin in the kidneys, which, in turn, is induced by hypoxemia (12). Erythropoietin stimulates the production of red blood cells in the bone marrow, causing erythrocytosis in peripheral blood. This increase in the amount of circulating erythrocytes produces an increase in blood viscosity, which can condition stasis and blockage of the capillaries. Symptoms of hyperviscosity usually appear when the hematocrit exceeds a value of 65% and are especially related to a progressive increase in hematocrit (13). Hyperviscosity syndrome, characterized by neurological and cardiopulmonary symptoms, can give rise to life-threatening complications, such as cardiac infarction, thromboembolic events, and ischemia in multiple organs (14).
Leukocyte count greater than 100,000/uL Hyperleukocytosis
is defined as a leukocyte count greater than 100,000/uL (15-16). This condition, in hematological pathologies such as acute myeloid leukemia and acute lymphoblastic leukemia, can produce a leukostasis syndrome. Leukostasis syndrome is characterized by neurological and cardiopulmonary symptoms and is considered a hemato-oncological emergency. In patients with acute myeloid leukemia, particularly, leukostasis is associated with a poor prognosis due to a high risk of early mortality and a high probability of long-term recurrence and death (16). Rapid cytoreduction by leukapheresis has been proposed as a therapeutic action in patients with leukostasis due to acute leukemia; this measure has been shown to reduce early mortality in patients with leukostasis (17-18).
Leukocyte count greater than 50,000/uL and a neutrophil count greater than 30,000/uL in neonates
A persistent leukocytosis greater than 50,000/uL not associated with leukemia is called leukemoid reaction, its main causes being infections, intoxications, tumors solids, severe bleeding and hemolysis (19-20). In neonates, the leukemoid reaction is defined by a white blood cell count greater than 50,000/uL or a neutrophil count greater than 30,000/uL and, in low birth weight patients, is associated with life-threatening conditions such as neonatal sepsis, intraventricular hemorrhage and bronchopulmonary dysplasia (21). Mortality due to neonatal sepsis has been estimated between 11% and 19% (22), and increases significantly in the case of multidrug-resistant microorganisms (23). Likewise, in our environment, mortality due to intraventricular hemorrhage in low birth-weight neonates has been estimated at 47.1% (24).
Neutrophil count less than 500/uL
Febrile neutropenia, a condition defined by a neutrophil count less than 500/uL in the presence of fever (temperature greater than 38°C on two consecutive readings), is a significant cause of morbidity and mortality in cancer patients (25). In adult cancer patients, in-hospital mortality associated with febrile neutropenia has been estimated at 9.5%, although the percentages are significantly higher in patients with major comorbidities, fungal infections, and sepsis (26). Likewise, in adult patients receiving myelosuppressive chemotherapy, febrile neutropenia is associated with a 15% higher mortality than in patients without febrile neutropenia (27), since it predisposes a severe infection by fungi, gram-negative bacilli, and gram-positive cocci (28).
In pediatric patients receiving myelosuppressive chemotherapy, febrile neutropenia is defined by a neutrophil count of less than 500 u/L or less than 1000/uL with a predicted decline over the next two days (29). In this group of patients, in-hospital mortality associated with febrile neutropenia is estimated at 5% for gram-positive infections, 18% for gram-negative infections, and higher percentages for fungal infections (30).
Platelet count less than 10,000/uL
Thrombocytopenia is defined as a platelet count of less than 150,000/uL (31, 32). Patients with platelets above 50,000/uL often have no symptoms or clinical findings; bleeding from minimal trauma or prolonged bleeding from wounds usually appears with values below 30,000/uL and spontaneous bleeding events, considered a hematological emergency, occur with values below 10,000/uL (31-32).
Platelet count greater than 1,000,000/uL
A platelet value greater than 1,000,000/uL is associated with acquired von Willebrand syndrome: deficiency of von Willebrand factor multimers caused by increased proteolytic activity of ADAMTS13 (33). The risk of bleeding is therefore increased in these extreme thrombocytoses. In patients with essential thrombocythemia, a platelet value greater than 1,000,000/uL, associated with a history of minor bleeding and a disease time greater than 15 years, is considered a high-risk factor for hemorrhage (34). Likewise, it is recommended to moderate or restrict the use of aspirin in patients with essential thrombocythemia who reach platelet levels above 1,000,000/uL (33-34).
Table 1. Critical values for automated blood counts .
| CRITICAL VALUES FOR AUTOMATED BLOOD COUNTS | ||
|---|---|---|
| PARAMETER | VALUES / FINDINGS | INTERPRETATION |
| Hemoglobin | < 7 g/dL | Risk of death in post-surgery. Recommended transfusion, with exceptions. |
| > 22 g/dL | Neonates: Definition of polycythemia. Risk of hyperviscosity. | |
| Hematocrit | > 65% | Hyperviscosity symptoms in heart patients. A possible indication of bleeding. Neonates: Definition of polycythemia. Exponential risk of hyperviscosity in neonatal polycythemia. Possible indication for exchange transfusion. |
| Leukocytes | > 100,000/uL | Risk of death due to leukostasis. Possible indication for leukapheresis. |
| > 50,000/uL (neonates) | Risk of death in neonatal leukemoid reaction. | |
| Neutrophils | < 500/uL | Risk of death associated with febrile neutropenia. |
| > 30,000/uL (neonates) | Risk of death in neonatal leukemoid reaction. | |
| Platelets | < 10,000/uL | Risk of death from spontaneous bleeding. |
| > 1,000,000/uL | Acquired von Willebrand factor deficiency, risk of bleeding, and aspirin contraindication. | |
CRITICAL VALUES FOR PERIPHERAL BLOOD Smears
Schistocytes
The International Council for Standardization in Haematology (ICSH), which we can translate as the International Committee for Standardization in Hematology, indicates that a percentage of schistocytes greater than 1% of the total red blood cells, in the absence of other anomalies severe erythrocytes, has clinical importance for the diagnosis of microangiopathic hemolytic anemia (35). Although a percentage of schistocytes greater than 1% can also be observed in renal failure, hemoglobinopathies, and neonates, it is usually accompanied by other erythrocyte morphological changes (36).
One of the most frequent microangiopathic hemolytic anemias in adults, thrombotic thrombocytopenic purpura (TTP), has mortality close to 90% in the absence of treatment (37). With the introduction of therapeutic plasma exchange (TPR) in the 1980s, mortality was reduced to values close to 20% (38-39). Due to the very high mortality of untreated TTP, TPR is considered to be indicated as an emergency procedure in these patients (39).
Blasts
The finding of blasts in peripheral blood, by itself, does not imply an imminent risk to the life of patients; however, its identification and morphological description are crucial for making therapeutic decisions, and a delay in its reporting can predispose to potentially serious damage.
For this reason, blasts fit the definition of “vital values”: outcomes that are just as important as critical values, but for which immediate corrective action is not required (2).
Although there is no standardized recommendation for reporting vital values, the need to include them as a logical extension of the critical values system has been recognized (40).
The Classification of Hematopoietic and Lymphoid Tissue Tumors of the World Health Organization (WHO) establishes a value of 20% of blasts in peripheral blood or bone marrow to define acute leukemia (41). Currently, however, we know that there is considerable intraindividual (the subject is compared against himself/herself at different times) and interindividual (the subject is compared against others) variability for microscopic examination and manual leukocyte count (42-43). Because of this, a blast count of less than 20% may be "corrected" in a second reading that shows a percentage above this threshold. Therefore, even a percentage of blasts below 20% should be reported in patients without a history of leukemia, with the promptness corresponding to a vital value.
Bacteria
Although the Wright stain, used in peripheral blood smears, is designed to identify leukocytes, red blood cells, and other cellular elements, it can also show microorganisms such as bacteria and parasites. The presence of bacteria in peripheral blood is a factor in poor prognosis and high mortality in patients with sepsis (44-45). This is a very unusual finding, so possible contamination of the sample with bacteria from the central venous catheter must be ruled out (45).
Hemoparasites
As in the case of blasts, a timely report of hemiparasites in peripheral blood is crucial for making therapeutic decisions; likewise, a delay in their identification could lead to serious complications. For example, our country's timely malaria diagnosis is a public health priority. The WHO emphasizes the early diagnosis and treatment of malaria patients to reduce incidence and mortality rates (46), a task in which clinical laboratories play a leading role. In this sense, a constant improvement has been verified, between 2012 and 2017, in the performance for the microscopic malaria diagnosis in the country's specialized laboratories (47). In this context, Dr. Pedro Legua (48) highlights the importance of timely malaria diagnosis and stresses the need to identify Plasmodium falciparum correctly.
Abnormal Promyelocytes
By “abnormal promyelocytes” (Figure 1) we mean promyelocytes with abnormal morphological characteristics that typically appear in the peripheral blood and bone marrow of patients with acute promyelocytic leukemia (APL). The WHO recognizes two morphological types of APL: the “classic” or hypergranular form and the microgranular or hypogranular variant (49). Additionally, other morphological types with their characteristics have been described (50).
Although the morphological presentation of APL is usually heterogeneous (50), in all cases, it is associated with a high early mortality rate that approaches 17.5% (51). This high mortality rate is related to the phenomenon of disseminated intravascular coagulation (DIC), which conditions events of hemorrhage and thrombosis (52), with intracranial hemorrhage being the leading cause of death from ALI (53). Therefore, starting treatment as soon as possible in patients with suspected ALI is recommended, even before the diagnosis is confirmed by molecular or genetic techniques (53).
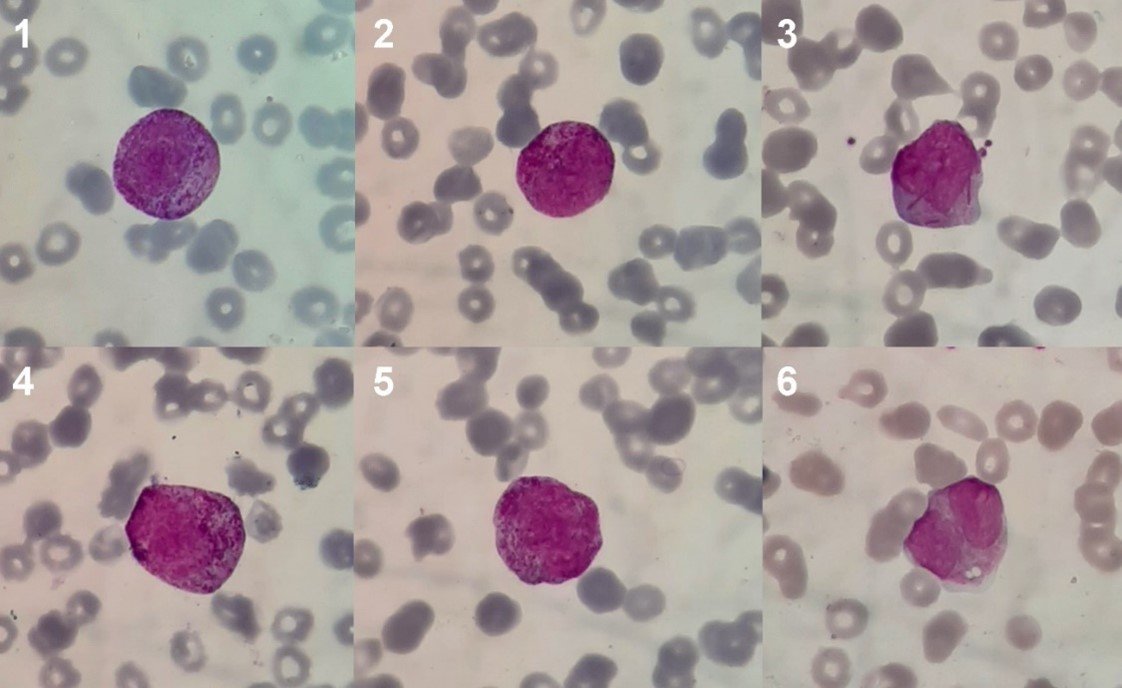
(Magnification: 100x. Coloration: Wright) Microphotographs 1-5: Anomalous prommyelocytes of hypergranular morphological type. Photomicrograph 3: Hypergranular promyelocyte with multiple Auer bodies in the cytoplasm. Microphotograph 6: Anomalous promyelocyte of microgranular morphological type or "variant".
Plasma cells
Plasma cells or plasmocytes (Figure 2) are lymphoid cells of the B lineage that originate in the bone marrow and whose function is to secrete antibodies. Although they constitute part of the normal cell population of the bone marrow, plasma cells are not usually found in the peripheral blood of healthy people (54). As we will see, the finding of plasma cells in peripheral blood is related, in some patients, to a poor prognosis and high mortality.
In patients with multiple myeloma, the presence of circulating plasma cells in peripheral blood is associated with a poor clinical course, poor survival, and an increase in the International Staging System (55). Similarly, in patients with multiple myeloma, a percentage greater than 5% of plasma cells in peripheral blood is considered a marker of a highly proliferative disease, low life expectancy, and a prognosis similar to that of plasma cell leukemia (56). Early mortality in plasma cell leukemia, although it has decreased in recent decades, it is still significantly higher than in multiple myeloma (57). This entity is defined by a plasma cell count greater than 20% of the total leukocytes in peripheral blood (58), and it is considered the most aggressive plasma cell neoplasm with the worst prognosis (59).
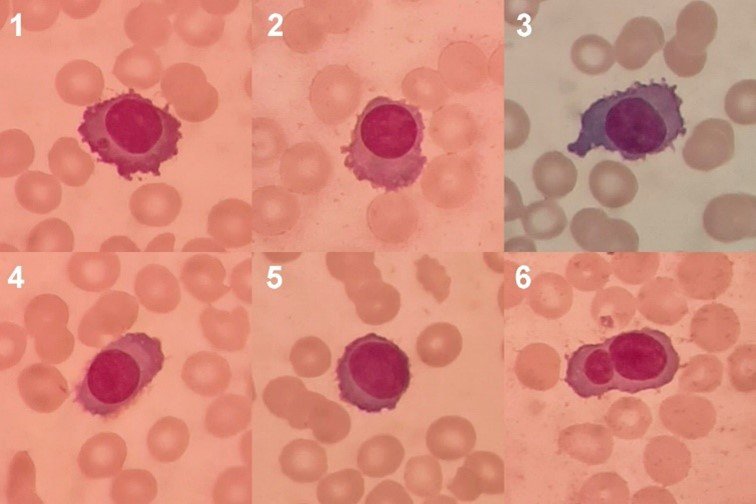
(Magnification: 100x. Coloration: Wright) Photomicrographs 1-6: Plasma cells with intensely basophilic cytoplasm with an irregular border, fine cytoplasmic processes, clear perinuclear zone, round or oval nuclei with eccentric location and closed chromatin. Microphotograph 1: A cytoplasmic inclusion is observed. Photomicrograph 5: Plasma cell with scant cytoplasm that could be mistakenly identified as an abnormal lymphocyte. Microphotograph 6: Binucleation is observed.
Table 2. Critical values for peripheral blood smears.
| CRITICAL VALUES FOR PERIPHERAL BLOOD SmearS | ||
|---|---|---|
| PARAMETER | VALUES / FINDINGS | INTERPRETATION |
| Schistocytes | > 1% of total red blood cells | Risk of death from thrombotic thrombocytopenic purpura. |
| Blasts | > 20% of total leukocytes (consider reporting lower percentages, according to clinical-pathological correlation). | Immediate communication of the finding can prevent potentially serious damage. |
| Bacteria | Any amount. | Very poor prognosis in patients with sepsis. |
| Hemoparasites | Any number on a peripheral blood smear. | Immediate communication of the finding can prevent potentially serious damage. |
| Abnormal promyelocytes | Predominant cell type, lacking later stages of granulocytic maturation (myelocyte, metamyelocyte, neutrophil). Correlation with other findings in the blood count and coagulation profile. | Risk of death from disseminated intravascular coagulation (DIC). |
| Plasma cells | > 5% of total leukocytes. | Risk of death in multiple myeloma. A value above 20% is the diagnostic criteria for plasma cell leukemia, with an aggressive course and poor prognosis. |
DISCUSSION
One of the most important considerations for reporting critical values, in the particular case of the hematology laboratory, is that a correct and thorough morphological review be carried out reasonably. For proper identification of some cellular elements and abnormal shapes, an experienced observer must examine peripheral blood smears.
For example, regarding thtitleon of schistocytes, the ICSH establishes that the morphology is variable (35): crescent-shaped schistocytes must be distinguished from sickle cells (sickle cells) based on their size, which in schistocytes is always smaller than a red blood cell; there are schistocytes with sharp angles or “spines” that look like triangles; “helmet cells”, damaged red blood cells with an “amputated” area recognizable by a rectilinear border, are considered to be equivalent to schistocytes; keratocytes, damaged red blood cells that appear to have a pair of "horns" separated by a concave segment, are also considered schistocyte equivalents; microspherocytes, likewise, should be included in the schistocyte count, but only in the presence of any of the previously mentioned forms.
The morphology of the abnormal promyelocytes of the LPA deserves a separate mention. Both morphological types mentioned by the WHO, the "typical" or hypergranular and the "variant" or microgranular, have specific characteristics that differentiate the abnormal promyelocyte from its normal counterpart (49).
Hypergranular promyelocytes have a cytoplasm filled with granules that range in color from pink to purple. These granules are compactly distributed in the cytoplasm and occasionally join or fuse (coalescence); they are usually comparatively larger than the granules of normal promyelocytes and may be so abundant they cover the nuclear rim, obscuring it. Other times, the granules are very fine and look like a "dust cloud" in the cytoplasm. In many cases, abnormal promyelocytes present multiple Auer bodies (rod-shaped, basophilic, crystalline cytoplasmic inclusions), which may adopt a bunch or bouquet distribution. The hypergranular promyelocyte nucleus is variable in size and shape, although it is usually bilobed or kidney-shaped (49-50).
Microgranular promyelocytes, in contrast, are characterized by an apparent absence of cytoplasmic granules and a nucleus that is usually bilobed. Not infrequently, however, a small number of abnormal promyelocytes with distinguishable granules and clustered Auer bodies are also seen in microgranular APL. Currently, we know that the apparent lack of granules is due to the fact that they are submicroscopic in size and cannot be seen by light microscopy (49-50).
Regarding plasma cells, since they are cells that are not normally observed in peripheral blood, special attention must be paid to correct morphological identification. The normal plasma cell is oval or circular, with abundant and intensely basophilic cytoplasm, a clear perinuclear zone corresponding to the Golgi apparatus, a low nucleus/cytoplasm ratio, and a small, eccentric nucleus with closed and lumpy chromatin. Additionally, in conditions such as multiple myeloma, plasma cells usually present atypical features in size, shape, and cytoplasmic coloration, different types of inclusions, abnormalities in the nuclear border, and multinucleation (60).
On the other hand, more and more importance is attached to the issuance of interpretive comments in laboratory tests; In our country, this responsibility corresponds to the medical specialist in clinical pathology. It is currently recommended that the laboratory results include a description or interpretation of the anomaly, information relevant to the diagnosis, suggestions for further examinations, and even an opinion related to the treatment (61). Critical value reporting is considered an integral part of the interpretive reporting system, providing the clinician with information about life-threatening conditions and prompting prompt and decisive therapeutic action.
CONCLUSIONS
The reporting of critical values is considered a necessary practice in laboratories and is beneficial in the clinical field. To have the desired impact, they need to be reported quickly, interpreted by a trained professional, and lead to prompt decision-making. Likewise, it is recommended that the critical values of each health establishment be chosen by consensus between laboratory professionals and those who work in clinical areas.
In the hematology laboratory, the critical values for complete blood counts comprise a series of quantitative parameters that, in most cases, are obtained directly from the automated hematology analyzer; in some cases, however, they can be corrected by the laboratory professional through a microscopic analysis (platelet count, leukocyte differential, etc.) or a manual procedure (microhematocrit by centrifugation, etc.). On peripheral blood smears, critical values include abnormal morphologic findings that often require evaluation by experienced professionals.
The recommendations proposed in this article can serve as a basis for other health establishments to prepare their own lists of critical values, considering that each laboratory service must do so in coordination with the interested clinical areas. Likewise, our recommendations can be used to expand the lists of critical values in those services that already have a notification system for these results.
ACKNOWLEDGMENTS
We thank Dr. Adela Fabiola Sáenz Bello, clinical pathologist, head of the Hematology, Hemotherapy and Blood Bank Service of the Alberto Sabogal Sologuren National Hospital, for having given us the facilities to carry out this review and for her constant support during its development. of our investigation.
Authorship contributions: the authors are the managers of the article in its entirety.
Funding sources: self-financed.
Conflicts of interest: The authors declare no conflict of interest .
Received: May 22, 2021
Approved: August 28, 2022
Correspondence: José Luis Huerto .
Address: Jr. Colina 1081, Bellavista, Callao. Lima Peru.
Telephone number: 965266908
E-mail: joluhuag@gmail.com
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
REFERENCES
