ARTÍCULO DE REVISIÓN
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i2.4730
Preoperative magnetic resonance imaging in locoregional breast cancer
Resonancia Magnética Preoperatoria En Cáncer De Mama Locoregional
Franklin Aldecoa Bedoya1,2,a,b, Maritza Placencia Medina3,c
1 Maestría en Medicina, Clínica Internacional San Borja
2 Universidad Peruana de Ciencias Aplicadas – UPC
3 Universidad Nacional Mayor de San Marcos
a Médico Especialista en Oncología Médica.
b Docente de la Facultad de Medicina.
c Doctora en Farmacia y Bioquímica.
ABSTRACT
Introduction. Preoperative magnetic resonance imaging (MRI) is controversial in patients with breast cancer, and there is no consensus on its benefit compared to standard images. The objective of this review was to evaluate the comparative studies of patients with non-advanced breast cancer, with or without the use of PROM. Methods. A search was done for medical articles published from January 1, 2000, to March 31, 2021, in MEDLINE/PUBMED, LILACS, and SCIELO, and publications that met the inclusion criteria were included. Results. There were 3,828 publications, of which 53 met the inclusion criteria; the selected articles were reviewed, and the results were organized in tables. There were 46 single- or multicenter retrospective and comparative studies, three prospective, randomized, controlled studies, and four meta-analyses that included patients with infiltrating ductal or lobular carcinoma and ductal carcinoma in situ. The comparative results were antagonistic and debatable; however, in the most relevant studies, it was shown that: PROM delays surgery; increases mastectomies and additional biopsies; increases detection of ipsilateral/contralateral disease not necessarily malignant; no significant difference was established in the rate of loco-regional or distant recurrence. Conclusions. MRI in non-advanced breast cancer has controversial results in the type of surgery, reoperations, and progression-free survival. It is necessary to have additional prospective, multicenter, randomized, and comparative studies that clearly define its role and benefit.
Keywords: Magnetic Resonance Image; Breast Neoplasms; Breast conserving surgery; Radical Mastectomy; Reoperation; Recurrence; Disease-Free Survival. (Source : MeSH - NLM).
RESUMEN
Introducción. El uso de la resonancia magnética preoperatoria (RMP) en pacientes con cáncer de mama es controversial y no existe consenso sobre su beneficio frente a las imágenes estándar. El objetivo de esta revisión, fue evaluar los estudios comparativos de pacientes con cáncer de mama no avanzado, con el uso o no de la RPM. Métodos. Se realizó la búsqueda de artículos médicos publicados desde el 01 de enero del 2000 hasta el 31 de marzo del 2021 en MEDLINE/PUBMED, LILACS y SCIELO y se incluyeron las publicaciones que cumplieron con los criterios de inclusión. Resultados. Hubo 3828 publicaciones, de las cuales 53 cumplieron los criterios de inclusión; se revisaron los artículos seleccionados y se organizaron los resultados en tablas. Hubo 46 estudios retrospectivos y comparativos uni o multicéntricos, tres estudios prospectivos, aleatorizados y controlados y cuatro metaanálisis que incluyeron pacientes con carcinoma ductal o lobular infiltrantes y carcinoma ductal in situ. Los resultados comparativos fueron antagónicos y discutibles, sin embargo, en los estudios más relevantes se demostró que: la RPM retrasa la cirugía; incrementa las mastectomías y las biopsias adicionales; aumenta la detección de enfermedad ipsilateral/contralateral no necesariamente maligna; no se estableció una diferencia significativa en la tasa de recurrencia loco-regional o a distancia. Conclusiones. La RMP en cáncer de mama no avanzado tiene resultados controversiales en relación al tipo de cirugía, reoperaciones y supervivencia libre de progresión, siendo necesario contar con estudios adicionales de tipo prospectivo, multicéntrico, aleatorizado y comparativo que defina claramente su rol y beneficio.
Palabras Clave: Imagen por Resonancia Magnética; Neoplasias de la Mama; Mastectomía Segmentaria; Mastectomía Radical; Reoperación; Recurrencia Local de Neoplasia; Supervivencia sin Enfermedad. (Fuente: DeCS BIREME).
INTRODUCTION
Breast magnetic resonance imaging (MRI) with dynamic images provides information on the transversal morphology of the lesion, functional characteristics, vascularization/perfusion, and permeability. This is the reason for its current nomenclature of Dynamic Contrast Enhancement Breast MR Imaging (DCE-MRI)(1-3). Breast MRI has evolved to high-resolution images that evaluate multiple parameters, unlike the initial conventional approach, which used only contrast-enhanced sequences to evaluate tumors. The interpretation must be made with radiologists experienced in breast images because, like all evolving technology, the learning curve requires sufficient time for greater certainty of the information(4).
The sensitivity of MRI in breast carcinoma is 88 to 100; its specificity reaches 72(5,6). Breast MRI is indicated according to the European Society of Breast Imaging in the detection of breast cancer in women at high risk, evaluation of the effect of neoadjuvant chemotherapy, evaluation of women with breast implants, occult primary breast carcinoma, suspicion of local recurrence, when needle biopsy cannot be performed, in troubleshooting (equivocal mammography/ultrasound findings) and preoperative staging of newly diagnosed breast cancer (ipsilateral and contralateral); however, the National Comprehensive Cancer Network in its most recent version of 2022, agrees with this guide, in some observations: detection of women with a risk > 20% of having a primary breast in their life; adds occult carcinoma of the breast, Paget's disease, and poorly defined invasive lobular carcinoma with other tools; finally, the preoperative staging places it in category 2B (based on low levels of evidence)(7,8).
The use of preoperative MR images in patients with breast cancer remains controversial. There is no consensus on whether it confers a benefit since it has not shown any advantages over standard images. Therefore, we need to know the real benefit that patients achieve in relation to the surgical decision based on this tool.
METHODS
Study inclusion criteria
- Meta-analysis; prospective or retrospective, single-center or multi-center clinical studies; Observational, retrospective clinical studies, with a control group, in patients with breast cancer (invasive, ductal carcinoma in situ and/or infiltrating lobular or ductal carcinoma), comparing preoperative MRI versus no MRI.
- Searched results. Rates of: lumpectomy or mastectomy, surgical reoperation, loco-regional or distant recurrence, disease-free or progression-free, and overall survival.
Study exclusion criteria.
- Clinical studies are evaluating breast cancer with histologies other than mammary adenocarcinoma.
- Clinical studies included patients with neoadjuvant treatment.
- Clinical studies included patients with metastases or patients with other synchronous cancers.
- Clinical studies prior to the year 2000.
Location and selection of relevant studies
The search was carried out from January 1, 2000, to March 31, 2021, with three different data engines: MEDLINE/PUBMED, LILACS, and SCIELO. MEDLINE/PUBMED was searched for all medical articles containing the word “Preoperative Magnetic Resonance AND Breast Cancer”; 3606 were found. In the LILACS platform and SCIELO, the words “Magnetic Resonance AND Breast Cancer” and “Magnetic Resonance AND Breast Cancer” were searched, 152 and 70 articles were found, respectively. Of the total collected, compliance with the inclusion and exclusion criteria was evaluated, and finally, 53 articles were selected for review. The information was transferred to tables designed to order the information based on the desired result.
RESULTS
All clinical studies presented two groups of patients: patients with preoperative MRI (MRI) and another group of patients with breast cancer who only used mammography and breast ultrasound, but not MRI (noMRI).
- .- Breast cancer (All histological types)
- Early breast cancer [Ductal carcinoma in situ (DCIS)]
- Early breast cancer (lobular or duct-lobular carcinoma)
Retrospective and comparative studies
Table 1 shows the retrospective, comparative studies between the RMP and noRMP groups. Fisher reported that the recurrence rate was 1/86 (1.2%) versus 9/133 (6.8%), and contralateral carcinoma was detected in 2/121 (1.7%) versus 9/225 (4%). ), both statistically significant(9). In contrast, Solin found no significant differences in the rate of loco-regional recurrence, overall survival, survival without metastatic disease, or in the presence of contralateral breast cancer in the RMP versus non-RMP groups(10).
Angarita in Canada and Grady in the USA found significant differences in the number of new tumors found in RMP patients vs. non-RMP(11,12). Other studies have shown a substantial increase in the initial and final mastectomies rate versus conservative surgeries (BCS) in the groups with MRI(11-19); however, other investigations have shown the opposite(20-26). Two studies found a significantly lower frequency of positive margins and rate of reoperations in patients with MRI versus non-MRI(27,28). Other studies found no significant differences(13,30).
Yi in South Korea found that patients with MRI had better ipsilateral locoregional recurrence-free survival than those without MRI(31). Hill in the United States found in a univariate analysis that locoregional recurrence was lower in patients undergoing MRI versus no MRI, with a mean follow-up of 8 years; however, multivariate analysis showed that MRI was not associated with loco-regional recurrence(32). In contrast, long-term studies such as the one by Ryu, Zeng, and Gervais with a follow-up of >5 and 10 years, did not show a significant difference in loco-regional recurrence-free survival in the groups with and without preoperative MRI(33-35). Finally, Onega analyzed a multicenter database (The Breast Cancer Surveillance Consortium) and showed that breast cancer-specific and adjusted mortality was not significant between both comparisons groups(36)
Meta-analysis of MRI in multifocal/multicentric breast cancer.
In 2008, a meta-analysis was published whose results showed that MRI detected the additional disease in 16% of women with breast cancer, the predictive prognostic value was 66%, and the ratio of true positives/false positives was 1 .91; conversion from a wide local excision to a mastectomy was 8.1%, showing that MRI in this context caused a greater extension of the surgery in a significant group of women(37).
Meta-analysis and prospective and randomized studies of MRI in breast cancer.
The first prospective, randomized, controlled, multicenter clinical study was published in 2010; 1623 patients with breast cancer were enrolled in 45 hospital centers in the United Kingdom. The COMICE study compared RMP (n=816), versus no RMP (n=807). It was shown that the use of MRI was not significantly associated with a reduction in the reoperation rate(38).
Subsequently, the MONET Study, with 211 patients in the noRPM group and 207 patients in the PRP group, showed that conservative surgery (BCS) was similar in both groups (68% versus 66%); Reoperations for positive margins after BCS were significantly higher in the MRI group versus the control group(39).
A meta-analysis published by Houssami and colleagues in 2013 found a significant initial mastectomy rate of 16.4% versus 8.1%; there was no difference in reoperation rate after BCS and overall mastectomy in the noRMP and RMP groups correspondingly(40).
In 2014, the POMB Study, which included 440 breast cancer patients under 56 years of age in Sweden, randomly assigned one group to RMP (n=220) and another to noRMP (n=220). The RMP group had a higher rate of BCS than the control group; however, there was a change in the mastectomy decision in 23/153 patients (15%). The reoperation rate was significantly lower in the MRI group: 11/220 (5%) versus 33/220 (15%) in the control group(41).
A new meta-analysis by Houssami in 2014 with 3169 patients showed that local recurrence-free survival at eight years was similar in patients with MRI (97%) versus non-MRI (95%); 8-year distant recurrence-free survival also did not differ between groups (89% versus 93%)(42).
Another meta-analysis published in 2017 by Houssami et al. included 19 studies: three prospective, controlled, randomized studies (COMICE, MONET, and POMB), and the rest were retrospective, comparative studies; 85,975 patients with and without MRI were included. The use of MRI was associated with a higher rate of mastectomy [OR: 1.39 (1.23, 1.57)]; there was no evidence of increased reoperation rates or positive margins; the MR group was more likely to receive contralateral prophylactic mastectomy [OR: 1.91 (1.25, 2.91)](43).
In 2015, a meta-analysis was published with 3,252 patients diagnosed with ductal carcinoma in situ (1,077 with RMP and 2,175 non-RMP); the MRI group was more likely to have an initial mastectomy (adjusted OR, 1.76). There were no significant differences in the proportion of women with reoperation after BCS(44).
In table N°2, the different retrospective studies that evaluate DCIS in the context of the use or not of MRI have been organized. The sensitivity of MRI allows the detection of a more significant number of tumors, for which Petrillo detected an additional 19.7% DCIS as opposed to conventional images; however, there were also 11.6% false negatives(45). Lam showed 30% of biopsies among patients who used MRI vs. 7% in those who did not; likewise, the number of surgeries was significantly higher(46). The results are quite controversial since other researchers found a higher proportion of mastectomies among patients who used RMP in relation to the group that only used conventional images(47-49); however, Davis demonstrated in a similar study that there was no significant difference in both groups(50-52).
Kropcho in 2011, found no significant differences in the finding of positive margins after surgery for DCIS, between the groups with and without MRI (24.7% vs 30.7%); there was indeed a difference in the rate of reoperation between both groups (17.7% vs 4.1%)(53). Yoon 2020 found a lower reoperation rate in the group that had MRI [OR: 0.33 (95% CI 0.12-0.92)](54). Contrary to these authors, Allen and So did not find significant differences in DCIS reoperation rates in these two groups of patients with and without the use of MRI(55,56).
Table 3 shows the retrospective; comparative studies carried out in the context of lobular or mixed-type breast cancer, that is, ductal and lobular (ductal) histology. -lobular), between patients who had RMP and those without RMP. The rate of mastectomy between both groups showed no statistical difference in four clinical trials conducted(57-60). Despite this, there is a trend towards a higher rate of reoperations in the clinical studies by Mann and Ha in the MRI group [OR: 3.29 (95% CI 1.22–8.85)(57) and (OR: 0.140)(60) and a tendency to be higher in the Moloney publication (38.0% vs 23.4%)(61).
Finally, Ha in 2019, in a single-center study, after a 9-year follow-up, found that the loco-regional recurrence rate for breast cancer with lobular or mixed components was not significant, nor was overall survival between RMP versus non-RMP.(62).
DISCUSSION
Most malignant breast neoplasms are adenocarcinomas, which constitute more than 95% of breast cancers and are classified as in situ or invasive. In carcinoma in situ, cells are restricted within the lobular-ductal system of the breast, whereas in invasive carcinoma, cells spread beyond that structure. Therefore, invasive carcinomas (both ductal, lobular or mixed) and ductal carcinoma in situ have been considered for this systematic review, but not lobular carcinoma in situ, since it is regarded as a non-obligate precursor of breast carcinoma(63,64).
In most of these studies, MRI patients were younger and had higher breast density. Premenopausal women are more likely to have aggressive breast tumor phenotypes as well as denser breasts than postmenopausal women(65). These biases can alter the results of the studies and lead to controversial conclusions.
The multifocality/multicentricity of breast cancer, evaluated in detailed pathological examinations of the excised breasts, ranges between 20% and 60%(66,67). Breast MRI improved sensitivity to reveal tumors not detected by other means; the first publications of MRI in breast cancer were based on observational studies. In this context, Kuhl published in 2007 that "breast MRI had shown to be very important in the local staging of breast cancer, allowing greater precision of tumor size and extension, detecting multifocal, multicentric or contralateral disease, intraductal extensions, making surgery more precise and avoiding unnecessary operations, which is why it should be used in the study of all patients who undergo conservation treatment for breast cancer”(68).
A 2014 meta-analysis, with 22 studies and 67,557 patients found the multifocal disease in 9.5% of cases; multivariate analysis showed lower overall survival (HR: 1.65) and trend towards worse disease-free survival (HR: 1.96) than a single disease; however, when studies with significant heterogeneity were excluded, there was no difference significant in overall survival(69). Yerushalmi found that the 10-year cumulative rate for local recurrence, in unifocal or multifocal/multicentric breast cancer treated with mastectomy (887 patients) versus BCS (300 patients), was 6.5% (58/887) versus 5.7% (17/300) respectively(70). A subsequent meta-analysis that included this last study concluded that the publications chosen for the systematic review were historical, of moderate quality, with little statistical power, limited follow-up, and selection biases that favored BCS instead of mastectomy in low-income patients. risk(71).
Many surgeons were more aggressive in multifocal/multicentric disease, which resulted in a higher number of mastectomies in the retrospective and comparative studies that we evaluated in Tables 1-3, in patients with MRI. BCS and mastectomy have shown a similar overall survival confirmed by two studies with a follow-up of 20 years(72,73), a perception, without solid scientific evidence, has led to the belief that mastectomy could be relevant in multifocal/multicentric disease. However, mastectomy is clearly based on a decision but does not categorically establish the benefit in the medium or long term benefit. To this we must add that many of these patients are subjected to complementary adjuvant treatments whose effects are not measured.
Positive margins and complementary reoperation after BCS were based on criteria of "sufficient margin" to avoid recurrence, and each institution created its own parameters to perform a reoperation. In 2014, the National Surgical Adjuvant Breast Projectconsidered that if the resection margin was free of cancer cells at the microscopic level, it was sufficient to avoid reoperation; there were different interpretations that led to an excess of reoperations (between 25% and 40%), with no pathological disease being found in about 50% of them(74). In 2016, the majority of academic institutions supported the "no tumor in the ink" as the definition of negative margin, thus reducing the rate of reoperations from 22% to 14%(75). It follows that the different publications shown in Tables 1-3, which evaluated the positive margins and the reoperation rate (obviously interrelated), had their results on a non-standardized basis and, therefore difficult to compare with each other, even so the trend was a higher rate of reoperations in patients who did not have MRI, which is added that the influence of the complementary treatment they received after surgery was not evaluated.
Probably, the results that best reflect the advantages of having or not having a MRI in early breast cancer are: loco-regional or distant recurrence rate, disease-free survival or local or distant recurrence, and finally, overall survival. Several studies have investigated ipsilateral recurrence rates in patients with early breast cancer with BCS associated with the use of adjuvant treatment, in invasive ductal, lobular, or mixed cancer, with 10-year recurrence rates ranging from 2.6% to 6.2%(76) and in ductal carcinoma in situ, with annual recurrence rates between 1.22% and 1.65%(77). Randomized, controlled, multicenter studies comparing lumpectomy alone versus lumpectomy plus radiotherapy have shown that the risk of local recurrence is significantly reduced by up to 70% over a 10-year period(78). In the retrospective and comparative studies of this review, antagonistic results were found that do not allow evaluating the true differential weight between the use or not of MRI, in early breast cancer.
Regarding the prospective, randomized and controlled studies, the first to be carried out was the COMICE(38) , which did not find that breast density significantly influenced the reoperation rate; One year later, the MONET(39) in patients with non-palpable breast lesions found that the reoperation rate was significantly higher in patients with MRI. Finally, the POMB(40) which was specifically designed for patients under 56 years of age, found that MRI resulted in a lower probability of requiring reoperation. However, these results are not measuring the interference of post-surgery treatments that have been shown to significantly reduce long-term recurrence rates. The fact that neither the COMICE study nor the MONET study showed any benefit for MRI was unexpected, however, Kestelman(79) maintains that both studies had a series of methodological limitations: inexperience in the use of MRI both at the level of radiologists and surgeons themselves, low reoperation rates without a consistent explanation, inexperience in taking MR-guided biopsies, among others.
In relation to meta-analyses, the first one carried out by Housami(37) in 2008 showed that MRI detected the additional disease in 16% of women with breast cancer, however, the ratio of true positives/false positives was 2:1, that is, out of every three women diagnosed and biopsied through MRI, one was false positive; conversion to mastectomy was 8.1%; it was one of the first studies to question the usefulness of the MRI. The same author published 2 more meta-analyses(41,43) showing that the use of MRI was associated with a higher rate of mastectomy, but not reoperation. There are criticisms of these meta-analyses on the basis that only three randomized trials were included and there were serious methodological deficiencies(80).
The intraluminal location of DCIS may generate doubts regarding the true limits of the tumor, which would allow conservative surgery, which is why it was thought that MRI could be helpful as a preoperative tool. A meta(45) in patients with DCIS showed that MRI does not improve the control of positive margins, nor the rate of reoperation. In one of the few studies that evaluated locoregional recurrence, Pilewskie(51) in 2321 patients with DCIS and lumpectomy showed that MRI did not have a significant impact on recurrence at 5 years.
Invasive lobular histology of breast cancer is known to be associated with greater difficulty in defining the extent of the breast tumor, which makes early detection difficult; Added to this is its propensity to spread to neighboring tissues and sometimes at a distance, which is why some doctors perceive them as tumors with poor results, despite the fact that most are hormone-dependent(81). Many researchers consider breast MRI a potential tool for planning breast conservation surgery, in this histological variety. The publication made by Ha(62) in 2019, with a 9-year follow-up, exposed this position when it found that loco-regional recurrence due to breast cancer with a lobular or mixed component was not significant in the groups with and without preoperative MRI.
The indications for MRI in breast cancer are clear and precise; its routine use in the preoperative evaluation of early breast cancer does not have high levels of evidence showing that it improves surgical planning and execution or that it reduces the number of surgeries, or more importantly, that it reduces local or distant recurrence or improves long-term survival(82).
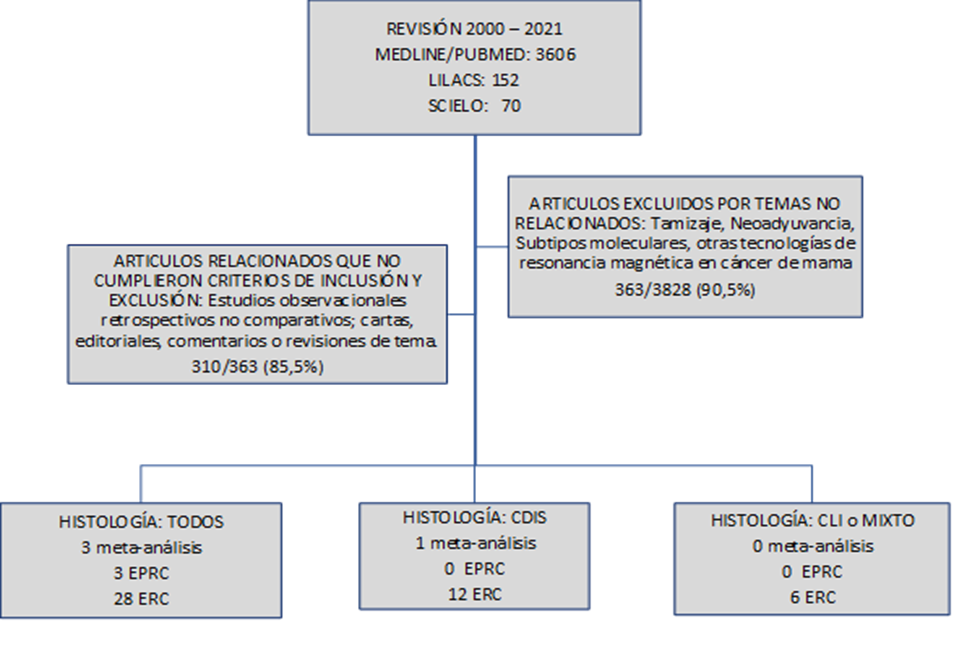
CDIS: Carcinoma Ductal in Situ
CLI: Carcinoma Lobular Invasivo
EPRC: Estudio Prospectivo, Randomizado y Controlado
ERC: Estudio Retrospectivo y Comparativo uni o multicéntrico.
Tabla 1. Estudios Observacionales, retrospectivos uni o multicentricos, comparativos (RM vs no-RM preoperatoria) en cáncer temprano de mama Invasivo y ductal in situ
| Autor/Año | Edad media (RM+ vs RM-) | N | RM % | D Tratamiento Quirúrgico | Mastectomia (RM+ vs RM-) | Margen positivo (RM+ vs RM-) | Reoperación (RM+ vs RM-) | Recurrencia RLR o RD (RM+ vs RM-) | Otros (RM+ vs RM-) | Observaciones |
|---|---|---|---|---|---|---|---|---|---|---|
| Fischer (Alemania) 2004 | 57,1 vs 55,2 | 346 | 121 (35%) | 87 (38,7%) vs 35 (28,9%) | RLR 6,8% vs 1,2% (p<0,001) | Carcinoma contralateral 4,0% vs 1,7% (p<0,001) | Seguimiento medio > 40 meses | |||
| Solin (USA) 2008 | 53,0 vs 56,0 (p=0,026) | 756 | 215 (28%) | RLR 3% vs 4% (p=0,51) | SG (86% v 87%, p=0,51). Metastasis (89% vs 92%, p=0,16), | Seguimiento: 8 años | ||||
| Pengel (Países bajos) 2008 | 56,1 vs 59,6 (p=0,02). | 349 | 173 (49,5%) | CCM a Mx 16/173 (9,2%) | 13,8% vs 19,4% (p=0,17) | Seguimiento medio 54,1 meses | ||||
| Bleicher (USA) 2009 | 52,5 vs 59,0 (p<0,001) | 577 | 130 (22,5%) | OR 1,80 (CI 95% 1,08–3,00, p=0,024) | 13,8% vs 21,6% (p=0,20) | Tiempo hasta cirugia 56,9 vs 38,1 días (p=0,010) | Pacientes con RM+ fueron más jóvenes (52,5 vs 59,0 años p<0,001), | |||
| Angarita (Canada) 2010 | 54,6 vs 64,4 (p< 0,0001) | 147 | 71 (48%) | Inicial 12% vs. final 26% | Tiempo hasta tratamiento. 36,0 vs 32,3 días (p=0,15). | |||||
| Weber (USA) 2012 | 53,6 vs 59,5 (p=0,001) | 313 | 120 (38%) | 47 (39,2%) vs 65 (33,7%) vs p=0,67 | Mx 10 (8,3%) vs 16 (8,3%) p= 0,92 | > Densidad mamaria MR+ (22,3% vs 68,4% p=0,001). | ||||
| Grady (USA) 2012 | 63,5 (31-99) | 184 | 79 (43%) | Cirugía adicional 11% vs 26% (p=0,04) | ||||||
| Wang (USA) 2013 | Por grupos | 36277 | 2554 (7%) | (37,4% vs 31,7%) p<0,0001 | Base de datos SEER-Medicare | |||||
| Obdeijn (Países Bajos) 2013 | 54,0 vs 55,2 (p=0,32) | 123 | 123 (100%) | 42 (34.1%). Mx: (29 , 23.6%). | 15,8% vs 29,3% p<0,01 | 18,9% vs 37,4% p<0,01. | Grupo histórico (control) | |||
| Petrillo (Italia) 2013 | 34,8 vs 34,7 (p=0.570) | 246 | 122 (50 %) | 437% vs 53% (p=0.011) | Pacientes menores de 40 años | |||||
| Killelea (USA) 2013 | 53 vs 60 (p<0,001) | 628 | 369 (59%) | 23% vs 26% p=NS; | Mx bilateral: 20% vs 12% <0,005 | |||||
| Sung (USA) 2014 | 52,0 vs 55,0 | 348 | 174 (50%) | 29% vs 45% p=0,02 | LRR (p=0,33) SLE (p=0,73) |
> densidad mamaria (28% vs 6%, p < 0,0001) | Tiempo medio seguimiento después de tto fue de 8 años | |||
| Fancellu (Italia) 2014 | 53,9 vs 56,4 (p=0,10) | 237 | 109 (46%) | 18/109 (16,5%) | 13,7% vs 7,0% (p<0,05) | 4,1% vs 8,6% (p=0,9) | Tamaño tumoral > RM+ 16,8& vs 13,9 mm (p <0,001). | |||
| Fortune-Greeley (USA) 2014 | Por grupos | 20332 | 2471 (12,2%) | OR: 1,33 (IC 95% 1,19–1,48) | Base de datos SEER-Medicare | |||||
| Parsyan (Canadá) 2015 | 55,3 vs 66,3 (p<0.001) | 765 | 307 | 20,5% vs 17,2%, (p=0,254) | 7,5% vs 8,7% (p=0,54) | Pacientes > 30 años | ||||
| Arnaout (Canadá) 2015 | Por grupos | 53,015 | 7824 (14,8%) | [OR: 1,73 (IC 95% 1,62-1,85)] | Mastectomia contralateral [OR:1,48 (IC 95% 1,23-1,77) | Base de datos de salud de Ontario, Canada | ||||
| Patel (USA) 2015 | 51,7 vs 51,7 (p=0,978) | 250 | 154 (62%) | 79 (51,3%) vs 39 (40,6%) p=0,100 | [OR = 4,33; (CI 95% 2,01-9,35; p < 0.001)] | |||||
| Yi (Corea del Sur) 2015 | 48,5 (20-89) | 742 | 371 (50%) | [HR: 0,15; IC 95% (0,07-0,32)]* | [HR: 0,03; IC 95% (0,04-0,21)] (p<0,001)** | *Supervivencia libre de RLR ipsilateral
** SLR mama contralateral |
||||
| Ozanne (USA) 2016 | Por grupos | 55997 | 9055 (16,2%) | 37,1% [OR: 1,04 (IC 95% 0,.98-1,11)] | [OR: 0,96 (IC 95% 0,89 - 1,03) | Base de datos SEER-Medicare | ||||
| Lai (Taiwan) 2016 | 52,2 vs 52,7 (p=0,31) | 1468 | 735 (50,1%) | 5,0% vs 9,0% (p<0,01). | 11,7% vs 3,2% (p<0,01) | |||||
| Gervais (Canada) 2016 | 56,8 (25-92 ) | 470 | 27 (5.8%) | 10 años: 1,6% vs 4,2% (p=0,37) | Seguimiento medio 97 meses | |||||
| Ryu (Corea del Sur) 2016 | 48,9 vs 50,5 (p=0,098) | 954 | 743 (77,8%) | SLRLC (p=0,938), SLE (p=0,507) | SG (p=0,655). | Seguimiento medio 64.5 meses MR+ ; 78.5 meses MR- | ||||
| Brück (Finlandia) 2017 | 61,0 vs 61,0 (p=0,617) | 100 | 50 (50%) | 10/50 (20%) | 6/50(12%) vs 2/50 (4%) (p=0,140) | 14% vs 24% (p=0,202) | Biopsias 14/50 (28%) 7/14 (50%) fueron malignos | Mayores de 35 anos. Estadío I | ||
| Hill (USA) 2017 | 60,0 vs 59,0 (p=0,12) | 1396 | 664 (47,6%) | 4,0% vs. 8,0% a 8 años (p=0,04)* | RM no asociado RLR [RR: 0,77 (0,45–1,28)]** | * Análisis univariado **Análisis multivariado |
||||
| Onega (USA) 2017 | Por grupos | 13097 | 2217 (16,9%) | 39,8% [OR: 1,32 (IC 95% 1,16-1,50)] | Mastectomia contralateral [OR: 1,32 (IC 95% 1,05-1,65)] | The Breast Cancer Surveillance Consortium (BCSC) | ||||
| Wang (USA) 2018 | Por grupos | 24379 | 4691 (19,2%) | 3,2 vs 4,1*[AHR: 0,92; IC 95% (0,70-1,19)] | Mortalidad 5,3 vs 8,7* [AHR: 0,89 (IC 95% 0,73-1,08)] | Base de datos SEER-Medicare *Por 1000 personas-año |
||||
| Onega (USA) 2018 | Por grupos | 4454 | 917 (20,6%) | Mortalidad* [HR: 0,90 (IC 95% 0,72-1,12) | *Específica y ajustada por cáncer de mama | |||||
| Zeng (USA) 2020 | 43,4 vs 43,6 (p=0,62) | 512 | 65% | 7.9 vs. 8.2%, p = 0.88 | RD 6.4 vs. 6.6%, p = 0.92 | Edad ≤ 50 Seguimiento 5.8 anos | ||||
| RM: Resonancia Magnética; D: Variación; RLR: Recurrencia Loco-Regional; RD: Recurencia a distancia; SG: Supervivencia Global; CCM: Cirugía Conservadora de mama o Tumorectomía; OR: Odds Ratio; Mx: Mastectomía; NS: No Significativo; SLRLC: Supervivencia Libre deRecurrencia Loco-Regional; SRL: Supervivencia libre de recurrencia; SLE: Supervivencia Libre de Enfermedad; HR: Hazard Ratio; AHR: Hazard Ratio Ajustado; IC 95%: Intervalo de confianza al 95%. | ||||||||||
Tabla 2. Estudios Observacionales, retrospectivos uni o multicentricos, comparativos (RM vs no-RM preoperatoria) en cáncer de mama ductal in situ
| Autor/Año | Edad media (RM+ vs RM-) | N | RM % | D Tratamiento Quirúrgico | Mastectomia (RM+ vs RM-) | Margen positivo (RM+ vs RM-) | Reoperación (RM+ vs RM-) | Recurrencia RLR o RD (RM+ vs RM-) | Otros (RM+ vs RM-) | Observaciones |
|---|---|---|---|---|---|---|---|---|---|---|
| Allen (USA) 2010 | 60,5 vs 64,4 | 99 | 64 (64,6%) | 20,3% vs 25.7% p=0,62 | 21,2% vs 30,8% p=0,41 | 21,2% vs 30.8% p=0,41 | ||||
| Itakura (USA) 2011 | 50,0 vs 59,0 (p<0,001) | 149 | 38 (25,5%) | 45% vs 14%, p<0,001 | MR+ tuvieron DCIS m+ás grandes (1,6 vs 1,0 cm; p=0,007) | |||||
| Kropcho (USA) 2011 | 55,0 vs 62,0 (p<0,001). | 158 | 60 (38%) | 17,7% vs 4,1% (p=0,004) | 24,7% vs 30,7% p = 0,414 | |||||
| Davis (USA) 2012 | ND | 218 | 154 (71%) | 8,9% vs 7,8% p=NS | 34% vs 39% p=NS | |||||
| Pilewskie (USA) 2013 | 53,0 vs 60,0 (p<0,0001) | 352 | 217 (61,2%) | 34,6 vs 27,4% p=0,20 | 14,3% vs 20,0% (p=0,19). | |||||
| Petrillo (Italia) 2017 | 63,3 vs 51,4 (p<0,001) | 362 | 117 (32,3%) | RM+ 19,7% DCIS adicionales y 11.6% de falsos negativos. | ||||||
| So (USA) 2017 | 56,4 vs 63,6 (p<0,001) | 176 | 97 (55,1%) | 28,9% vs 26,6% p=0,87). | ||||||
| Keymeulen (P. Bajos) 2019 | 50,0–74,0 (84,7%), | 10173 | 2382 (22,9%) | [OR: 2,11 (IC 95% 1,91-2,33)] | [OR: 0,99 (IC 95% 0,85-1,16)] | Mx secundaria [OR: 1,17 (IC 95% 1,00-1,37)] | *Registo Cáncer de Holanda < 75 anos | |||
| Lam (USA) 2019 | 55,7 vs 53,8 (p=0,29) | 373 | 332 (89%) | Biopsia 30% vs. 7% p=0,002; N° cirugías 1,2 vs 1,5 p<0,001 | ||||||
| Balleyguier (Francia) 2019 | 56,0 vs 58,0 | 352 | 178 (50,6)% | 18% vs 17% (p=0,93] | [OR: 0,68 (IC 95% 0,41-1,1; p=0,13)] | |||||
| Shin (Corea del Sur) 2019 | 54,0 vs 49,0 (p=0,001) | 541 | 430 (79,5%) | 3% (Apropiado 54%) | [OR: 0,39 (IC 95% 0,16-0,93 p=0,03) | [OR: 0,33 (IC 95% 0,12-0,92 p=0,03) | ||||
| Lamb (USA) 2020 | 60,2 vs 50,6 (p<0,001) | 963 | 236 (24,5%) | 34,3% vs 19,4% p<0,001 | 36,0% vs 37,4%p=0,85 | |||||
| RM: Resonancia Magnética; D: Variación; RLR: Recurrencia Loco-Regional; RD: Recurencia a distancia; OR: Odds Ratio; Mx: Mastectomía; NS: No Significativo. | ||||||||||
Tabla 3. Estudios Observacionales, retrospectivos uni o multicentricos, comparativos (RM vs no-RM preoperatoria) en cáncer temprano de mama lobular o mixto
| Autor/Año | Edad media (RM+ vs RM-) | N | RM % | D Tratamiento Quirúrgico | Mastectomia (RM+ vs RM-) | Margen positivo (RM+ vs RM-) | Reoperación (RM+ vs RM-) | Recurrencia RLR o RD (RM+ vs RM-) | Otros (RM+ vs RM-) | Observaciones |
|---|---|---|---|---|---|---|---|---|---|---|
| Mann (Países bajos) 2010 | 56 vs 61 (p=0,001) | 267 | 99 (37%) | 48% vs 59% p=0,098 | [OR: 3,29 (IC 95% 1,22–8,85). | |||||
| Heil (Alemania) 2011 | 57,8 vs 63,6 p=0,003 | 178 | 92 (52%) | 38% vs 30% p=0,119 | Cirugía bilateral 14 vs 3 patientes; p=0,002). | |||||
| Sinclair (Escocia) 2016 | ND | 138 | 59 (42,8%) |
40.70% | 32% vs 30% p=0,71 | |||||
| Ha (Corea del Sur) 2018 | 49,9 vs 51,5 p=0,035 |
603 | 369 (61,2%) | 25,5% | Mx inicial (OR: 0,876; p=0,528) | 39.3% vs 65.5% | (OR: 0.140; P<0.001) | Mx final (OR: 0,744; p=0,151) | ||
| Ha (Corea del Sur) 2019 | 48,6 vs 50,6 p=0,036) | 287 | 120 (41,8%) | RLR (HR:1,204; p=0,796) | SG (HR: 0,485; p=0,231) | |||||
| Moloney (Irlanda) 2020 | 56,4 vs 65,6 (p<0,001). | 218 | 70 (32.1%) | 38,0% vs 23,4% (p=0,057). | ||||||
| RM: Resonancia Magnética; D: Variación; RLR: Recurrencia Loco-Regional; RD: Recurencia a distancia; SG: Supervivencia Global; OR: Odds Ratio; Mx: Mastectomía; NS: No Significativo; HR: Hazard Ratio. | ||||||||||
CONCLUSIONS
Up to now, there is no clear evidence of the benefit of preoperative MRI in patients with locoregional breast cancer; Research studies that have evaluated the rate of mastectomies versus lumpectomies, the reoperation rate, loco-regional recurrence, and progression-free survival have controversial results. Additional prospective, multicenter, randomized, comparative, and well-designed studies are needed to better define the role of preoperative MRI in locoregional breast cancer.
Authorship contributions: The authors declare that the study presented is original, there is no ethical responsibility or data confidentiality. No informed consent or right to privacy was required.
Financing: Self-financed.
conflicts of interest: The authors declare that they have no conflict of interest.
Received: January 14, 2022
Approved: March 5, 2022
Correspondence: Franklin Aldecoa Bedoya
Address: Calle Mariel 190 Dpto 403 Urbanización Chacarilla del Estanque. Surco. Lima-Perú.
Telephone number: +51 938 159 635
E-mail: franklin.aldecoa@yahoo.com
REFERENCES
