ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i3.4915
SAFETY AND PROTECTIVE EFFECTS OF CENCHRUS ECHINATUS ON INDUCED BREAST CANCER IN RATTUS RATTUS
SEGURIDAD Y EFECTO PROTECTOR DE CENCHRUS ECHINATUS EN EL CÁNCER DE MAMA INDUCIDO EN RATTUS RATTUS
Cesar Braulio Cisneros Hillary1,2,a, Jorge Luis Arroyo Acevedo3,b, Maria Paula Bedoya Castle4,c, Betsy Alexandra Lazarus Huaman4,c, Katia Elizabeth Mendoza Chavez5,d, Evelin Juline Swans Hillary4,e
1Research Institute in Biomedical Sciences, Ricardo Palma University. Lima, Peru.
2Cesar Vallejo University, Chimbote- Peru.
3Faculty of Medicine "San Fernando", National University of San Marcos, Lima-Peru.
4Faculty of Human Medicine, Ricardo Palma University. Lima, Peru.
5Peruvian Association of Science, Technology and Healthy Environment. Chimbote, Peru.
aMaster of Pharmacology with Mention in Experimental Pharmacology
bDoctor of Pharmacy and Biochemistry
cUndergraduate Student
dMastering in Clinical and Health Psychology
eObstetrician
ABSTRACT
Introduction: Breast cancer is a disease that is increasing its incidence yearly; it is thus in search of complementary alternatives for treatment, some medicinal plants containing large amounts of polyphenols have been studied, which have anti-cancer effects as is the case of Cenchrus echinatus L. Objective: determine the safety and protective effect of the extract ethanolic of Cenchrus echinatus L. (cadillo) on 7,12-Dimethylbenzo[a]anthracene DMBA-induced breast cancer in Rattus rattus. Methods: Preclinical, experimental study in male Balb/C53 mice and Holtzman rats of both sexes. Acute toxicity (DL50) and toxicity at 45 days were calculated by methods of the probits and OECD respectively; to evaluate the protective effect the method of Barros 2004 was used, also the proliferation of tumor cells was recorded microscopically, using descriptive and inferential statistical analysis, considering p<0.05. Results: The safety studies demonstrate that the extract does not induce significant changes at the hematological, biochemical, and anatomopathological levels. A 76.92 % of the protective effect of the extract against DMBA-induced breast cancer in rats was achieved. Conclusions: It has been shown that the extract of Cenchrus echinatus L. presents a protective effect on 7,12-Dimethylbenzo[a]anthracene-induced breast cancer in Rattus rattus, and is not toxic to mice and rats.
Keywords: Cenchrus echinatus L.; Breast cancer; DMBA. (fuente: MeSH NLM).
RESUMEN
Introducción: El cáncer de mama es una enfermedad que va aumentando su incidencia anualmente; es así que en búsqueda de alternativas complementarias para el tratamiento, se han estudiado algunas plantas medicinales que contienen grandes cantidades de polifenoles, los cuales tienen efectos anticancerígenos como es el caso del Cenchrus echinatus L. Objetivo: Determinar la seguridad y el efecto protector del extracto etanólico de Cenchrus echinatus L. (cadillo) sobre el cáncer de mama inducido por 7,12-Dimetilbenzo[a]antraceno DMBA en Rattus rattus. Métodos: Estudio preclínico, experimental en ratones machos Balb/C53 y ratas Holtzman de ambos sexos. Se calculó la toxicidad aguda (DL50) y la toxicidad a 45 días mediante métodos de los probits y OECD respectivamente; para evaluar el efecto protector se utilizó el método de Barros 2004, también se registró microscópicamente la proliferación de células tumorales, utilizando el análisis estadístico descriptivo e inferencial, considerando p<0.05. Resultados: Los estudios de seguridad demuestran que el extracto no induce cambios significativos a nivel hematológico, bioquímico y anatomopatológico. Se logró un 76.92 % del efecto protector del extracto frente al cáncer de mama inducido por DMBA en ratas. Conclusiones: Se ha demostrado que el extracto de Cenchrus echinatus L. presenta efecto protector sobre el cáncer de mama inducido por 7,12-Dimetilbenzo[a]antraceno en Rattus rattus; y no es tóxico en ratones y ratas.
Palabras Clave: Cenchrus echinatus L.; Cáncer de mama; DMBA. (fuente: DeCS BIREME).
INTRODUCTION
Cancer is a disease that has been increasing its incidence in developed and developing countries, expecting an increase of cases of 47% (28.4 million) between 2020-2040, due to risk factors other than only socioeconomic or modifiable factors such as overpopulation but also non-modifiable factors such as age (1-4).
This global expansion, positions cancer as the leading cause of premature death, achieving a decrease of up to 3 years in life expectancy in countries such as Norway and Western Europe(5). The Global Cancer Statics 2020, reported the occurrence of 19.3 million new cases of cancer and nearly 10 million deaths due to the same; it was also checked that breast cancer cases surpassed lung cancer with 2.3 million new cases (11.7%) against 11.4% respectively, although lung cancer remains the neoplasm with the highest mortality with 1.8 million deaths followed by colorectal, liver, stomach and breast cancer (4).
Global studies in breast cancer have demonstrated that with standardization of age to 45.9 mortality and disability-adjusted life years (AVAD) have increased; also by means of the sociodemographic index (SDI) it was found that mortality decreased in females between the age ranges of 15-49 and 50-69 (6). Likewise, its incidence decreased in regions with high SDI and increased considerably in areas with medium and low SDI (7).
Early detection of breast cancer in women plays a fundamental role, as a late diagnosis would favor progression of the neoplasm to advanced stages such as III or IV(8). The causative factors of the delay in diagnosis are education, marital status, not recognizing the signs or symptoms of the disease, the fear of death, and not having health services nearby (9-11).
In several countries the possibility of employing medicinal plants as a complement or alternative treatment has opened up low cost and easily accessible in certain regions, being Peru a megadiverse country in terms of plant species some researchers recognize their therapeutic contributions as it is a case of Morinda citrifolia, Annona muricata, Uncaria tomentosa, Cenchrus echinatus L, demonstrating anticancer properties (12-15).
It has been found that the ethanolic extract of Cenchrus echinatus L. possesses tannins, phenolic compounds, alkaloids, flavonoids, and free amino acids in higher proportion, and in lesser amounts of quinones and glycosides(13-15). Associating polyphenols as responsible for antiulcer, cardioprotective, hepatoprotective(14), hypolipidemic(15), anticancer(16-18) and antioxidant(19-20).
In 2000 the OECD (Organization for Economic Cooperation and Development) recommended as necessary the determination of acute toxicity and mean lethal dose (DL50) of medicinal products(21-22). Possible spontaneous changes in rodent behavior are thus also evaluated(23). Being a harmless product when DL50 values exceed 5 000 mg/Kg (23-25).
There are wild species with mutagenic, estrogenic, and immunosuppressive properties among which we found DMBA and N-Methyl-N-nitrosourea (NMU) (26-28). Whereas DMBA induces the appearance of an average of 4.7 hormone-dependent mammary tumors in mice, with histopathologic features and genetic alterations of the neoplasia described in humans (29).
At present, there are a limited number of plant products studied, their components, the determination of their therapeutic effectiveness, and the dosage to use. For such reasons we set out to determine the safety and protective effect of the ethanolic extract of Cenchrus echinatus L. (cadillo) on DMBA-induced breast cancer in Rattus rattus.
METHODS
Design and study area
An observational, prospective type experimental study with a longitudinal approach was conducted.
Population and sample
Two rodent populations (Mus musculus and Rattus rattus var albinus) were worked with, being the samples conformed by 84 male Balb/C53 albino mice (25 ± 5 g body weight) for the determination of DL50; 20 Holtzman rats (10 male and 10 female rats of 160 ± 20 g body weight) for evaluating the toxicity at 45 days and 50 female Holtzman rats (80 ± 10 g body weight), for the evaluation of breast anticancer effect, all specimens proceeded from the bioterium of the National Institute of Health Lima-Chorrillos.
Variables and instruments
For the variable Cenchrus echinatus L. extract, oral safety at a single dose (DL50) in mice and oral safety at repeated doses in rats were evaluated, also a phytochemical screening of the extract was considered. While for the breast cancer variable breast cancer induction was performed in mice with DMBA and the effect of Cenchrus echinatus L. extract, administered orally for 16 weeks was evaluated. The instruments were conformed by collection sheets from where body masses, number of deaths (DL50) were recorded; body masses, soft organ mass, blood biochemistry (repeat dose safety), types and amounts of secondary metabolites (phytochemical screening); number, size, and location of breast tumors, blood biochemistry (Protective effect of the extract against breast cancer).
Procedures
Collection of the plant: The plant sample, whole plants of Cenchrus echinatus L. were collected in the village of San Jose, district of Santiago de Cao, Province of Ascope. Department of Liberty.
Taxonomic identification: The plant sample was classified according to the Cronquist system from the year 1988 at the Museum of Natural History of the National University of San Marcos (Constancia N° 140-USM-2010).
Obtaining the ethanolic extract: The samples were selected, washed, and dehydrated at 40ºC in an oven with circulating air, then pulverized in a hand mill and macerated with ethanol at 96° at room temperature for 7 days; subsequently, the solvent was filtered and eliminated on a stove at 40°C, until a constant weight was obtained. The residue obtained was called ethanolic extract, which was preserved in amber flasks at 4oC (30)
Phytochemical study: A battery of tubes containing 1 mL of an alcoholic solution of Cenchrus echinatus L. extract was prepared to which the reactions of Gelatin, Ferric trichloride, Dragendorff, Molisch, NaOH 10%, Sulfuric vanillin, Liebermann, Shinoda, and Ninhydrin, to determine the presence and amount of secondary metabolites (31).
Determination of lethal dose 50 (DL50): 14 randomized groups of six mice were formed in each group, and the treatments were administered orally and only once, the first group received physiological serum solution 5 mL/kg (SSF), and the other groups ethanolic goat extract at doses of 10, 100, 500, 1,000, 5,000, 7,500, 10,000, 12,500, 15,000, 17,500, 18,750, 19,375, and 20,000 mg/kg of body weight respectively. The mice were observed during the first 24 hours and subsequently daily within 14 days, noting the toxic symptomatology, weight, and a number of deaths, the DL50 was calculated by making use of the Probits statistical method (24-32) .
Determination of oral toxicity at 45 days: It was carried out keeping in mind OECD-1995 trial 407, 20 Holtzman strain rats (ten of both sexes) were used, four groups of five rats were formed, which received SSF 5 mL /Kg and ethanolic caddy extract 100 mg/kg orally, during the 45 days, a weekly control of the weights of the rats was taken, finally anesthetized with sodium pentobarbital 30 mg/Kg intraperitoneally (VI) and se a 5 mL blood sample was withdrawn by cardiac puncture, which served to determine the bleeding, then the rats were euthanized with 100 mg/kg sodium pentobarbital VI and underwent a laparotomy to remove the soft organs and their macroscopic evaluation. Experimental design for determining the protective effect of the extract: 50 female albino rats were used randomly distributed in five groups of ten rats each group: The 1st group received SSF 5 mL/Kg, 2nd group DMBA 20 mg/rat ( 33), the 3°, 4°, and 5° groups received DMBA, in addition to the extract at doses of 10, 100 and 200 mg/Kg respectively, all treatments were administered orally (VO) making use of a metal cannula.
Induction of the tumor masses: DMBA was used, which was administered at the beginning of the experiment, by VO at a single dose of 20 mg/mouse (33), using 1 mL of olive oil as a vehicle, the weekly control was taken from weight of the rats, as well as, the number, size and location of the tumor masses, during four months, the same which were compared numerically and percentage with the control group that received DMBA 20mg/Kg, using the following formula: % inhibition of tumor masses = ((CT)/C) *100, where C=control, T=treatment.
The anatomopathological analysis: At week 16, the rats were euthanized with an overdose of sodium pentobarbital (100 mg/Kg) administered VI, and breast tumor masses were extracted for macro and microscopic evaluation, which were preserved in 10 al formol % for his anatomopathological study.
Statistical analysis
The statistical program SPSS version 17 was used, which allowed us to determine stratigraphy and assess differences and homogeneity (Kolmogorov-Smirnov test) between groups. The comparison of the means was done with the help of the Student’s t-test, Chi-square, and analysis of variance, among others. For the different analyzes, p<0.05 was considered. Mean body weight values at repeated doses are compared by a nonparametric method, the Mann-Whitney U test.
Ethical aspects
The studies were conducted in compliance with international standards, regarding the handling of laboratory animals. The whole procedure was conceived as stipulated in trial 407 of the OECD Guidelines (34-39) .
RESULTS
In Table 1, it can be appreciated that the ethanolic extract of Cenchrus echinatus contains tannins, phenolic compounds, alkaloids, flavonoids, and free amino acids in abundant amounts, quinones, and glycosides in regular amounts, without identifying the presence of triterpenic steroids.
Table 1. Phytochemical study of Cenchrus echinatus L.
| Identification Reaction | Secondary Metabolite | Amount |
|---|---|---|
| Gelatin | Tannins | +++ |
| Ferric Trichloride | Phenolic Compounds | +++ |
| Dragendorff | Alkaloids | +++ |
| Mayer | Alkaloids | +++ |
| Sodium | Hydroxide Quinones | ++ |
| Alpha naphthol | Glycosides | ++ |
| Liebermann | Steroids and triterpenes | - |
| Shinoda | Flavonoids | +++ |
| Ninhydrin | Free amino acids | +++ |
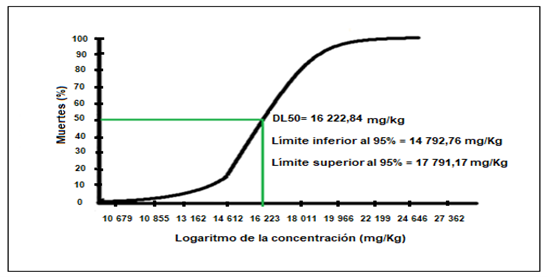
In Figure 1, the number of deaths of mice due to oral administration of increasing concentrations of ethanolic extract of Cenchrus echinatus L. from 10 mg/Kg up to 20 000 mg/Kg of weight, is observed being the DL50 (dose causing death of 50% of the specimens) of 16 223 mg/Kg.
In Table 2, a. Blood biochemistry values evaluated during the oral safety assessment at 45 days of the ethanolic extract of Cenchrus echinatus L., represented by: Total cholesterol, High-density lipoprotein (HDL), triglyceride, glucose, urea, Pyruvic Glutamic Transaminase, are shown (TGP), alkaline phosphatase. Also in b. Leukocyte numbering and formula values are evidenced represented by the amount of: Stocked, segmented, eosinophils, basophils, monocytes, lymphocytes and leukocytes. Similarly in c. It shows the weights of rat soft organs such as stomach, spleen, kidney, liver, lung, heart and brain. All parameters are shown considering the measured value with their respective standard error, the treatment groups were conformed by male (M) and female (H) rats, which received SSF 5 mL/Kg and 100 mg/kg of Cenchrusechinatus L respectively.
Tabla 2. Título 2 de la tabla lorem impsum.
| 2a. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTO | Total cholesterol (mg/dL) p<0,701 | HDL (mg/dL). p<0,655 |
Triglycerides (mg/dL). p<0,782 |
Glucose (mg/dL). p<0,860 |
Urea (mg/dL) p<0,809 | TGP (U/L) p<0,415 | Alkaline phosphatase (UI/L) p<0,743 | |||||||
| VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | |
| SSF (M) | 156,8 | ± 9,5 | 43,2 | ± 2,1 | 150,0 | ± 7,2 | 88,9 | ±3,3 | 16,2 | ±2,2 | 24,0 | ±5,8 | 150,0 | ±16,6 |
| Ext (M) | 165 | ±12,6 | 43,8 | ± 2,7 | 156,8 | ± 9,4 | 89,5 | ±4,0 | 17,2 | ±2,2 | 29,8 | ±7,2 | 127,6 | ±15,8 |
| SSF (H) | 166,6 | ± 5,0 | 41,2 | ± 1,1 | 159,3 | ± 4,1 | 92,6 | ±4,2 | 19,8 | ±2,9 | 26,8 | ±4,4 | 136,4 | ±16,4 |
| Ext (H) | 173 | ± 9,8 | 40,4 | ± 2,5 | 152,4 | ± 4,6 | 89,4 | ±5,0 | 17,8 | ±2,2 | 23,4 | ±2,7 | 138,6 | ±4,3 |
| 2b. | ||||||||||||||
| TTO | Stopped (%) p<0,407 | Segmented (%) p<0,665 | Eosinophils (%) p<0,225 | Basophils (%) p<o,418 | Monocytes (%) p<0,366 | Lymphocytes (%) p<0,857 | Leukocytes (U/mm3) p<0,473 | |||||||
| VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | |
| SSF (M) | 1,4 | ± 0,4 | 59,2 | ± 2,0 | 0,6 | ± 0,4 | 0,0 | ±0,0 | 1,6 | ±0,4 | 37,2 | ±1,6 | 8434,0 | ±549,3 |
| Ext (M) | 2,4 | ± 1,0 | 57,4 | ± 1,9 | 2,4 | ±0,9 | 0,0 | ±0,0 | 0,6 | ±0,2 | 37,2 | ±3,4 | 7 924,0 | ±751,8 |
| SSF (H) | 0,6 | ± 0,4 | 61,0 | ± 2,5 | 2,0 | ± 1,0 | 0,0 | ±0,0 | 1 | ±0,3 | 35,4 | ±3,0 | 8060,0 | ±951,1 |
| Ext (H) | 1,8 | ± 0,9 | 62,0 | ± 2,3 | 0,6 | ± 0,2 | 0,2 | ±0,2 | 0,8 | ±0,2 | 34,6 | ±1,9 | 8308,0 | ±885,9 |
| 2c. | ||||||||||||||
| TTO | Stomach p<0.456 | Spleen p<0.024 | Kidney p<0.000 | Liver p<0.001 | Lung p<0.120 | Heart p<0.019 | Brain p<0.000 | |||||||
| VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | |
| SSF (M) | 4,9 | ± 0,8 | 1,6 | ± 0,2 | 1,2 | ± 0,2 | 10,0 | ±0,4 | 1,9 | ±0,3 | 1,0 | ±0,1 | 1,5 | ± 0,0 |
| Ext (M) | 4,2 | ± 0,4 | 1,0 | ± 0,1 | 0,9 | ± 0,1 | 10,5 | ±0,7 | 1,8 | ±0,2 | 1,1 | ±0,1 | 1,6 | ± 0,0 |
| SSF (H) | 3,9 | ± 0,7 | 0,9 | ± 0,1 | 1,1 | ± 0,0 | 9,7 | ±0,2 | 1,5 | ±0,2 | 0,7 | ±0,1 | 1,5 | ± 0,0 |
| Ext (H) | 4,8 | ± 0,5 | 0,8 | ± 0,1 | 1,1 | ± 0,1 | 10,3 | ±0,5 | 1,6 | ±0,2 | 0,9 | ±0,1 | 1,7 | ± 0,0 |
In Table 3Table 3, The parameters considered when evaluating the protective effect of the ethanolic extract of Cenchrus echinatus L. at week 16 of experimentation are shown as follows: 3a. blood biochemistry represented by: Total cholesterol, High-density lipoprotein (HDL), triglyceride, glucose, urea, Pyruvic Glutamic Transaminase (PGT), alkaline phosphatase. Also in 3b. Leukocyte numbering and formula values represented by: Stored, segmented, eosinophils, basophils, monocytes, lymphocytes and leukocytes are evident. All parameters are shown considering the measured value with their respective standard error, the treatment groups were conformed by female rats.
Tabla 3. Evaluation of the protective effect of ethanolic extract of Cenchrus echinatus L. in rats 3a. Blood biochemistry parameters. 3b. Leukocyte numbering and formula.
| 3a. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTO | Total cholesterol (mg/dL) p<0,786 | HDL (mg/dL). p<0,910 |
Triglycerides (mg/dL). p<0,571 |
Glucose (mg/dL). p<0,205 |
Urea (mg/dL) p<0,809 | TGP (U/L) p<0,000 | Alkaline phosphatase (UI/L) p<0,024 | ||||||||
| VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | ||
| SSF | 158,3 | ±10,1 | 50,0 | ± 5,5 | 118,8 | ±10,6 | 84,8 | ±2,2 | 14,0 | ±1,5 | 12,8 | ±0,9 | 105,5 | ±9,8 | |
| TX | 171,4 | ± 5,9 | 49,8 | ± 2,6 | 141,0 | ±10,2 | 93,6 | ±2,5 | 18,5 | ±1,6 | 27,0 | ± 2,8 | 139,4 | ±11,4 | |
| TX-E10 | 164,0 | ± 10,5 | 54,2 | ± 3,2 | 134,0 | ±10,8 | 85,8 | ± 5,0 | 14,2 | ±1,2 | 17,2 | ±2,4 | 136,4 | ±4,2 | |
| TX-E100 | 162,7 | ± 7,2 | 50,1 | ± 3,6 | 137,4 | ±8,0 | 86,1 | ±3,2 | 16,2 | ±0,5 | 13,8 | ±1,3 | 118,6 | ± 6,2 | |
| TX-E200 | 160,1 | ± 8,4 | 52,4 | ±3,0 | 133,4 | ±6,2 | 84,3 | ±3,5 | 14,8 | ±1,3 | 14,1 | ±1,7 | 108,4 | ± 5,2 | |
| 3b. | |||||||||||||||
| TTO | Stopped (%) p<0,408 | Segmented (%) p<0,502 | Eosinophils (%) p<0,469 | Basophils (%) p<o,705 | Monocytes (%) p<0,284 | Lymphocytes (%) p<0,742 | Leukocytes (U/mm3) p<0,171 | ||||||||
| VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | VM | EE | ||
| SSF | 0,7 | ± 0,3 | 63,8 | ± 3,9 | 1,3 | ± 0,5 | 0,0 | ±0,0 | 0,3 | ±0,3 | 33,1 | ±4,0 | 5 293 | ± 251 | |
| TX | 1,6 | ± 0,5 | 62,7 | ± 2,5 | 2,3 | ± 0,9 | 0,0 | ±0,0 | 1,8 | ±0,6 | 33,2 | ±3,1 | 7 470 | ± 764 | |
| TX-E10 | 1,2 | ± 0,6 | 56,2 | ± 3,9 | 2,2 | ± 0,8 | 0,0 | ±0,0 | 0,6 | ±0,2 | 38,6 | ±3,2 | 6 982 | ± 822 | |
| TX-E100 | 0,7 | ± 0,3 | 62,6 | ± 3,1 | 1,3 | ± 0,8 | 0,1 | ±0,1 | 1,2 | ±0,4 | 34,9 | ±3,3 | 6 822 | ± 468 | |
| TX-E200 | 1,2 | ± 0,3 | 59,3 | ± 1,9 | 0,7 | ± 0,3 | 0,1 | ±0,1 | 1,2 | ±0,4 | 37,1 | ±1,8 | 6 269 | ± 452 | |
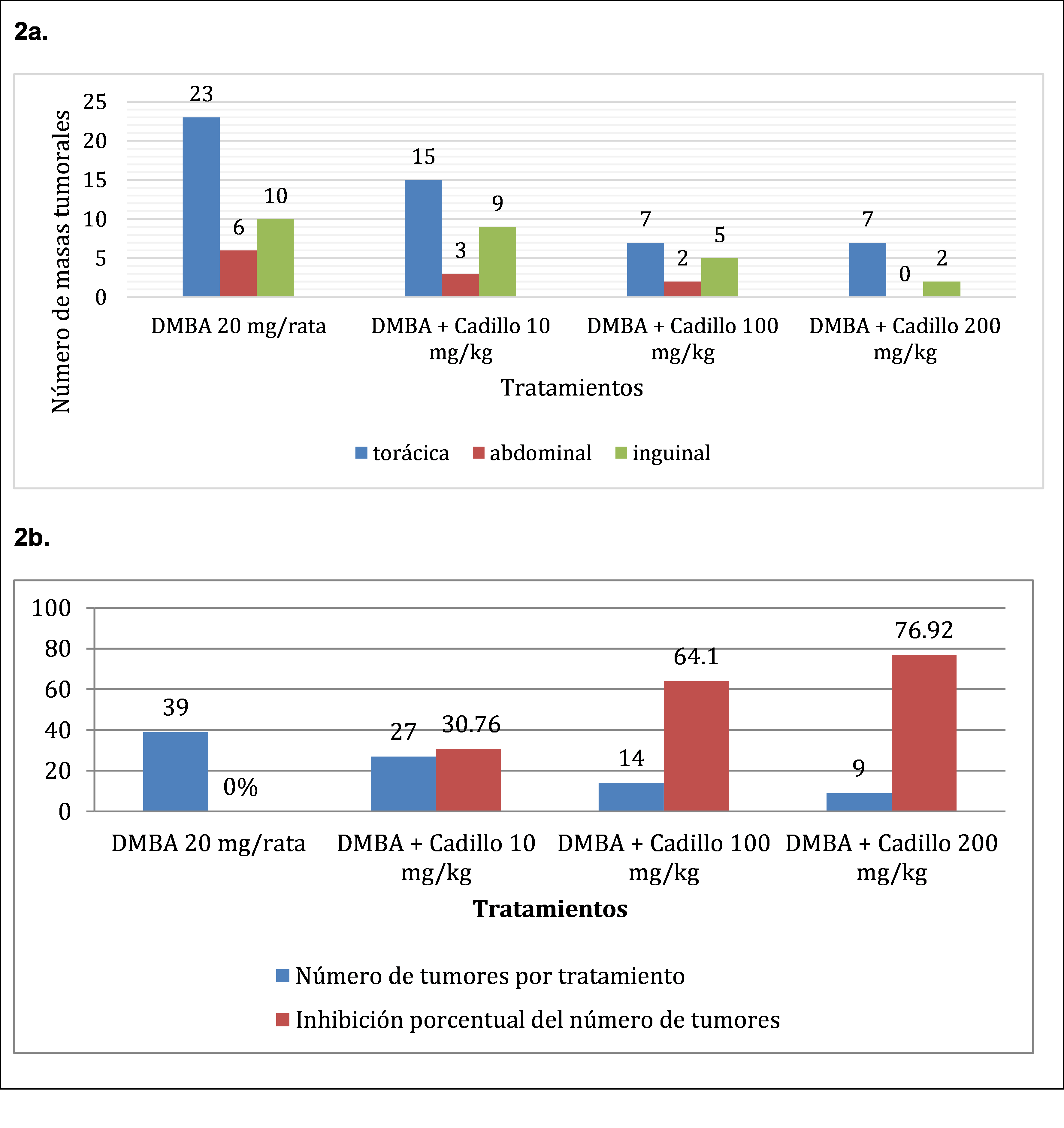
In Figure 2, 2a. a higher amount of tumor mass formation is appreciated in the DMBA 20 mg/mouse group (39 units), distributed 23 at the thoracic level, six at the abdominal level and ten at the inguinal level, as well as a lower tumor mass formation in the group who received DMBA + Cadillo 200 mg/Kg with a total of nine tumor masses distributed seven at the thoracic level and two at the inguinal level. It is also observed in 2b. In relation to the group that received the DMBA 20 mg/mouse (39 tumor masses) and the DMBA + goat 200 mg/kg group (nine tumor masses) there is a 76.92% percentage inhibition of tumor masses.
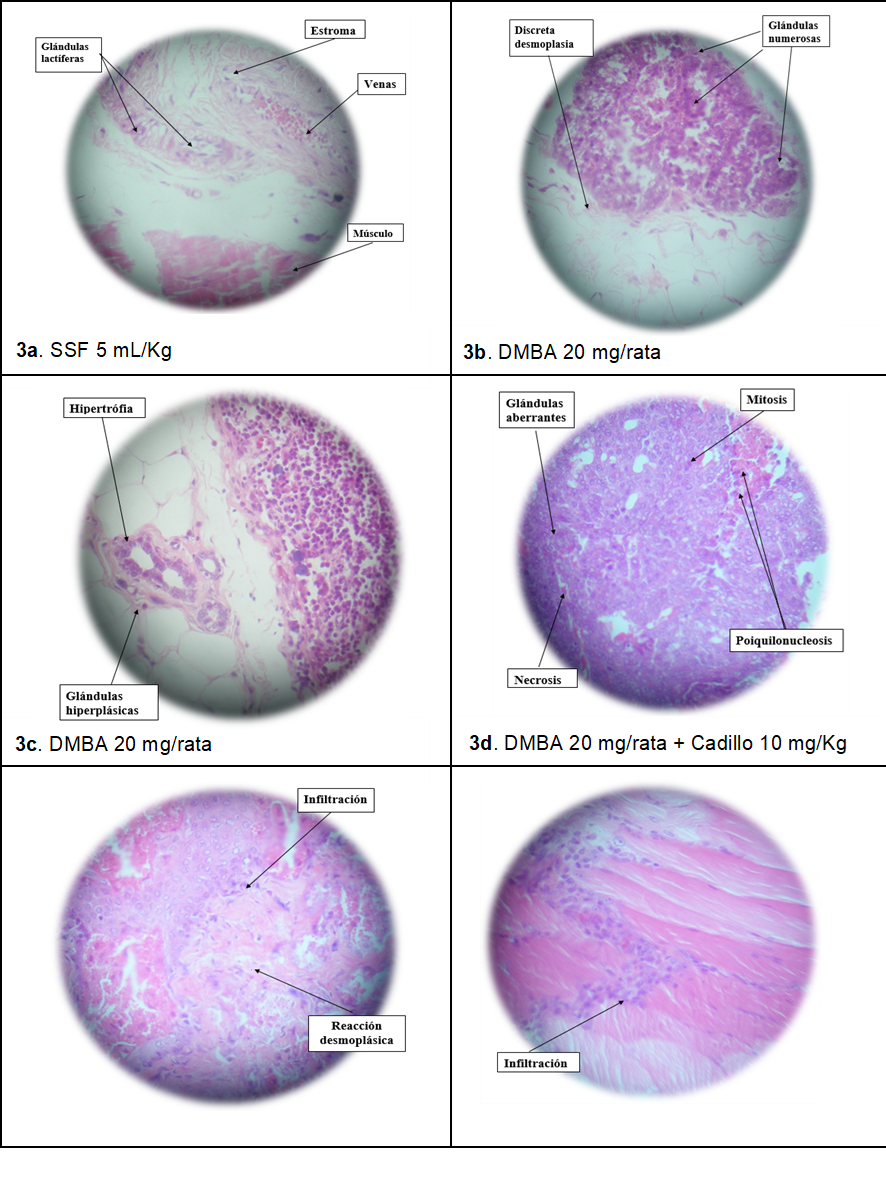
In Figure 3. The anatomopathological study of the tumor masses is observed: 3a. Normal mammary gland; 3b and 3c. Hyperplastic zones and mammary adenocarcinoma. 3d. Breast adenocarcinoma with metastasis. 3e. Mammary gland with inflammatory infiltration. 3f. Mammary gland with lymph node plus infiltration of mononuclear cells in muscle zone.
DISCUSSION
Cenchrus echinatus L. is an annual plant, native to America, of the family Poaceae, of caespitose nature with erect or decumbent, branched and glabrous stems 15–85 cm high, can be found on beaches, disturbed sites, tropics and subtropics; at an altitude of 0–760 meters (40).
The phytochemical study of the extract evidenced the presence of abundant amount of flavonoids, which besides presenting low toxicity is considered a safe use plant product, with protective activity on breast cancer, as well as antioxidant, anti-inflammatory properties, among others (41) .
When evaluating the oral toxicity of the ethanolic extract of Cenchrus echinatus L., it was observed, a median lethal dose (DL50) of 16 223 mg/kg, besides a sustained increase in weights of the mice from 27 – 33 g for 14 days, failing to evidence signs of toxicity or death of mice at doses above 5000 mg/kg orally, considered virtually nontoxic(42). In addition, according to European Community standards (2000) it classifies acute oral toxicity, as non-toxic when the dose of 2000 mg/kg is exceeded. Being able to compare this with OECD Guideline 407 where in one study two well-known immunosuppressants, azathioprine (AZA) and cyclosporine A (CysA), were chosen as model compounds in a dose range that did not cause visible toxic signs in animals during a treatment period of 28 days (43).
In the evaluation of oral safety at 45 days it was found that Cenchrus echinatus L. extract did not interfere with the development of albino rats as the body weights of the specimens increased in a gradual manner in all groups from 78-170 g; the blood biochemistry values (Total cholesterol, HDL, glucose, urea, TGP, alkaline phosphatase, supplied, segmented, eosinophils, basophils, monocytes, lymphocytes and leukocytes) and the macroscopic study of the soft organs (Stomach, spleen, kidney, liver, lung, heart and brain) showed no important alterations, the variations found are within the range established in the CENPALAB, 2010 for this species (44-45).
DMBA is an aromatic, highly lipophilic polycyclic hydrocarbon, which, to cause breast carcinogenicity requires its bioactivation and interaction with DNA, becoming epoxides and generating increased free radical formation, where lobes one and two of the gland mammary present the highest cell proliferative index and hence have more metabolic activity, which remains confirmed with our results since we observed a higher proliferation of tumor masses in the group receiving only DMBA (4 tumors per mouse), locating the highest proliferation at thoracic level.
On the other hand, estrogens are known to be responsible for breast cancer initiation and promotion; thus, inhibition of estrogen synthesis by lutein (4-tetrahydrochalcone) may be effective in breast cancer prevention by strongly inhibiting aromatase. Adding that possibly, some substances present in the Cenchrus echinatus L. extract may have favored the findings obtained in the present study.
Our main finding was the protective efficacy of the ethanolic extract of Cenchrus echinatus L., when a decrease in the appearance of tumor masses was evidenced from 39 units with DMBA to nine units with Cenchrus echinatus L 200 mg/Kg. Being compared with a study conducted in 32 21-day-old female Sprague Dawley rats, to which N-methyl-N-nitroso urea (NMU) was administered intraperitoneally, they found that sirolimus treatment decreased the expressions of ER and PgR of breast cancer already in turn reduced the size of tumor masses (46).
In a study where NMU was employed as a breast cancer inducer and sirolimus as treatment, the histology of the tumor masses presented no histologic subtypes of aggressive invasive carcinoma of no special type (NST) (46), whereas in our study one was evidenced epithelial and myoepithelial proliferation of the cells in most of the breast tumors, showed an anticancer efficacy of the extract of 30.76%, 64.10% and 76.92% at doses of 10, 100 and 200 mg/Kg of caddy extract respectively, whose protective effect is attributed to the presence of flavonoids that induce apoptosis by activating caspase 8 and Bax, inhibiting Bcl-2 expression and allowing cytochrome C release (47).
Therefore, breast cancer being a major health problem globally, with higher frequency in women, the search for new safe and effective products to tackle this disease becomes necessary(29).
The results of this research work presented its limitations in terms of observing the behavior of experimental animals during the assessment of the oral safety of the extract, as well as conducting long-term safety studies such as chronic toxicity, with the administration of treatments by other routes of administration and the incorporation of parameters such as reactive oxygen species in blood, as well as antioxidant and antiangiogenic activity of the extract could support the present study.
CONCLUSION
The ethanolic extract of Cenchrus echinatus L was obtained, which considering important secondary metabolites such as flavonoids, phenolic compounds and tannins in higher amount is considered as safe use plant product and besides would have implications on breast cancer.
Under experimental conditions it has been shown that the ethanolic extract of Cenchrus echinatus L. administered orally for 16 weeks is protective against 7,12-Dimethylbenzo[a]anthracene-induced breast cancer in female rats, upon evidence of a decrease in the number of y size of the tumor masses, as well as a greater protection from the ethanolic extract of Cenchrus echinatus L. at a dose of 200 mg/Kg.
Authorship contributions: César Braulio Cisneros Hilario: Conception and design of the article, analysis, and interpretation of data, drafting of the article, critical revision of the article, approval of the final version.
Jorge Luis Arroyo Acevedo: Design of the article, analysis, and interpretation of data; critical revision of the article, approval of the final version.
María Paula Bedoya Castillo: Collection of results, drafting of the article, critical review of the article.
Betsy Alexandra Lázaro Huamán: Collection of results, drafting of the article, critical review of the article.
Katia Elizabeth Mendoza Chavez: Collection of results, drafting of the article.
Evelin Juline Cisneros Hilario: Collection of results, drafting of the article.
Funding sources: Self.
Conflicts of interest: The authors declare no conflict of interest presented.
Received: April 28, 2022
Approved: June 07, 2022
Correspondence: César Braulio Cisneros Hilario.
Address: Urb. The Sunflowers Of The Mill Mz. G Lot 24 The Mill, Lima, Peru.
Telephone number: 993976156
E-mail: cbraulio.cisnerosh@gmail.com
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
REFERENCES