ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i3.5043
CLINICAL IMPLICATIONS OF THE MOLECULAR BIOLOGY OF PROSTATE CANCER: REVIEW ARTICLE
IMPLICANCIAS CLÍNICAS DE LA BIOLOGÍA MOLECULAR DEL CÁNCER DE PRÓSTATA: ARTÍCULO DE REVISIÓN
María del Carmen Castro-Mujica1,a
1Faculty of Human Medicine, Ricardo Palma University, Lima-Perú.
aMedical geneticist.
ABSTRACT
To understand the term genomic heterogeneity in prostate cancer, we must understand the clonal genomic evolution of cancer, as well as know that it is a dynamic and evolutionary phenomenon. Knowing the genome of prostate cancer not only allows us to have a vision over time of the genomic alterations, that occur during its different stages, but also to learn about the mechanisms of metastasis. In addition, knowing the hereditary component of prostate cancer allows us to evaluate patients and be able to identify if we are dealing with a family at risk.
Keywords: Prostate Neoplasms; Genetic Heterogeneity; Germ Line Mutation. (Source: MeSH NLM).
RESUMEN
Para enteder el término de heterogeneidad genómica en cáncer de próstata debemos comprender la evolución genómica clonal del cáncer, así como saber que es un fenómeno dinámico y evolutivo. Conocer el genoma del cáncer de próstata no solo nos permite tener una visión en el tiempo de las alteraciones genómicas que se producen durante sus diferentes estadíos, sino también conocer sobre los mecanismos de la metástasis. Además, conocer el componente hereditario del cáncer de próstata permite la evaluación de los pacientes y poder identificar si estamos frente a una familia en riesgo.
Palabras Clave: Neoplasias de la Próstata; Heterogeneidad Genética; Mutación de Línea Germinal. (fuente: DeCS BIREME).
INTRODUCTION
Prostate cancer is, from a molecular point of view, biologically heterogeneous due to the diversity of inter and intratumor molecular alterations since it is part of a dynamic and evolutionary genomic process. With the development of new molecular techniques, such as gene sequencing, microarrays, and epigenomic studies, among others, it has been possible to molecularly characterize prostate cancer, finding differences even between the different stages of the disease.
In addition to molecular alterations at the somatic level in prostate tumor cells, there are also variants at the germ line level, that is, they are present in all the cells of your body from the moment of conception, giving them a higher risk of developing prostate cancer throughout life and at a younger age. Men who have a family history of prostate cancer, mainly first-degree, are more likely to develop the disease as it could be a hereditary syndrome.
The objective of this review article is to present molecular alterations at the somatic level in prostate cancer, its molecular classification, genomic heterogeneity, clonal evolution, biomarkers, as well as genetic predisposition syndromes to prostate cancer.
MOLECULAR ALTERATIONS AT THE SOMATIC LEVEL
The main molecular alterations described in prostate cancer include the TMPRSS2–ETS gene fusion, copy number variants of TP53, AR, RB1, PTEN/PIK3CA, BRCA2, and ATM genes, among others.
• TMPRSS2–ETS gene fusion
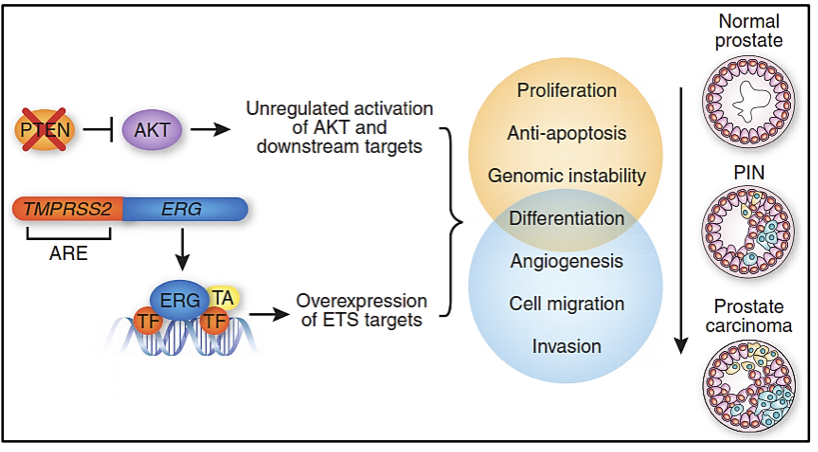
TMPRSS2 (Transmembrane protease, serine 2) is an androgen-regulated serine protease. ETS (erythroblastosis virus E26 oncogene homolog) is one of the largest families of transcription factors that includes ERG and ETV1, which are fused with the TMPRSS2 gene in approximately 50-79% of prostate cancer cases (1). This fusion is due to the deletion of a region between both genes and is associated with a worse prognosis in localized cases (2).
• PTEN/PI3K/AKT/mTOR pathway
The PTEN/PI3K/AKT signaling pathway plays a primordial role in the regulation of cell growth and death, while the PI3K/AKT/mTOR pathway plays a fundamental role in tumor metastasis (3). Variants in the PTEN and PI3K genes are mutually exclusive since the PTEN gene is a negative regulator of the PI3K/AKT pathway, so the loss of PTEN is associated with a worse prognosis (1). 2-14% of prostate cancer cases have variants in the PTEN gene and 12-41% of cases have copy number loss (4). On the other hand, variants in the PI3K gene have been described in 3-4% of prostate cancer cases and its amplification has been reported in 4-10% of cases. In addition, cases of combined variants of PTEN and TP53 have been described, which are very aggressive (4).
• TP53
The TP53 gene is a tumor suppressor that plays a very important role in maintaining genomic stability and preventing carcinogenesis. Approximately 3-47% of prostate cancer cases have variants in the TP53 gene and between 2-15% gene loss (4). Variants in the TP53 gene have been associated with an increased risk of disease recurrence (1).
• AR
About 2-18% of prostate cancer cases have variants in the androgen receptor AR gene (androgen receptor gene) or gene amplification (5-52% of cases), being very common in cases refractory to hormonal therapy (4). Androgen receptors belong to the group of nuclear steroid hormone receptors and act as a ligand-dependent transcription factor, that controls the expression of specific genes (1).
• RB
The RB protein is a product of the RB1 gene (Retinoblastoma 1 gene), a tumor suppressor which is mutated in approximately 1-4% of prostate cancer cases, while loss of the RB1 gene has been shown in 5-23% (4). An alteration in the RB1 gene leads to a failure in the function of the protein, stimulating the release of the androgen receptor and conferring resistance to castration (1). The RB1 gene is frequently deleted or methylated in castration-resistant prostate cancer (1).
• APC
• The APC gene is a tumor suppressor that encodes a protein that acts antagonistically to the Wnt signaling pathway (1). Hypermethylation of the APC gene promoter is a predictor of poor prognosis in prostate cancer. Approximately 3-10% of prostate cancer cases have variants in the APC gene (4).
• MYC
The MYC proto-oncogene encodes a protein that plays an important role in cell cycle progression, apoptosis, and transformation (1). Activation of this proto-oncogene transforms it into an oncogene that is expressed through gene amplification, thus stimulating cancer development, which is found in approximately 2-20% of prostate cancer cases (4).
• BRCA2
BRCA2 is a tumor suppressor gene, which is responsible for maintaining genomic stability, mainly in the double-stranded DNA repair pathway by homologous recombination (1). Approximately 9% of prostate cancer cases present variants in BRCA2, of which 2-6% are due to germline variants, which are a prognostic factor for survival in all stages of prostate cancer, including localized cases (5). When the variants are germinal, the relatives of the patient have a greater probability of having inherited the mutated gene, conferring risk of developing a neoplasm related to hereditary breast and ovarian cancer syndrome in carriers.
• ATM
• ATM is a DNA repair gene that acts at the cell cycle level as a necessary controller for the cellular response to DNA damage and to maintain genomic stability (1). Approximately 5% of prostate cancer cases have somatic ATM variants, including 1% germline variants (5).
TMPRSS2-ETS GENE FUSION
In 2005, Scott Tomlims et al. identified a recurrent rearrangement in more than half of the prostate cancer cases analyzed, which led to the gene fusion of the TMPRSS2 gene (21q22) with members of the ETS transcription factor family (ERG, ETV1, and ETV4 located at loci 21q22, 7p21 and 17q21, respectively)(6-8). Assessing the presence of the TMPRSS2-ETS gene fusion in prostate cancer samples serves as a prognostic marker of the disease. Determination of ERG overexpression by immunohistochemistry has been highly correlated with gene fusion status with 86% sensitivity and specificity (6).
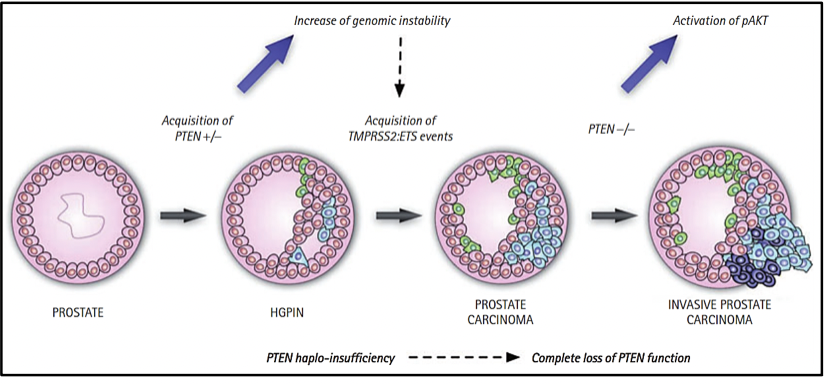
MOLECULAR CLASSIFICATION OF PROSTATE CANCER
There is currently a molecular classification of primary prostate cancer. All seven molecular subtypes of primary prostate cancer, considered the earliest drivers of carcinogenesis, are defined by ERG fusions (46%),
ETV1/ETV4/FLI1 fusions or overexpression (8%, 4%, 1%, respectively), or by variants in the genes SPOP (11%), FOXA1 (3%) and IDH1 (1%) (9-11), while 26% is made up of other variants. Likewise, it has been determined that the molecular profiles in primary and metastatic prostate cancer are different.
GENOMIC HETEROGENEITY IN PROSTATE CANCER
The heterogeneity can be intratumor and intertumor, as well as in the different stages of prostate cancer, which is why we currently speak of molecular subtypes in prostate cancer. The high tumor heterogeneity has implications for the diagnosis, follow-up, and treatment of patients with prostate cancer (12).
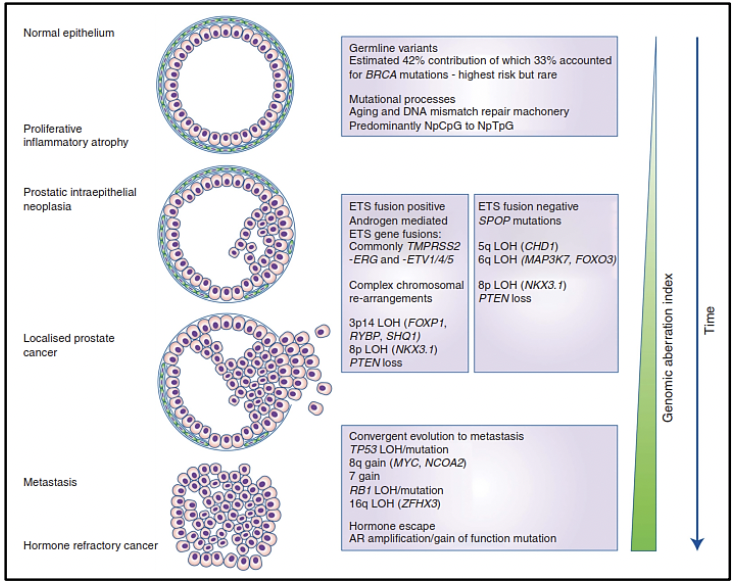
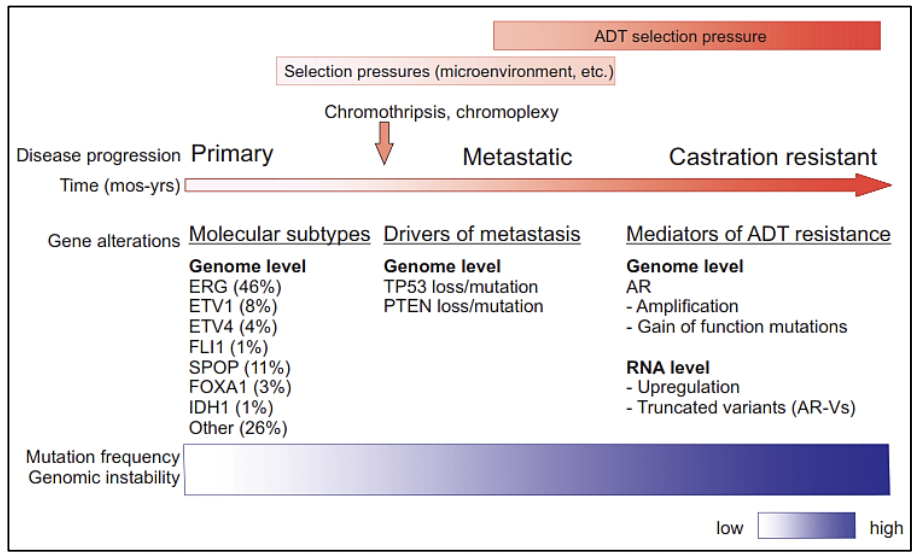
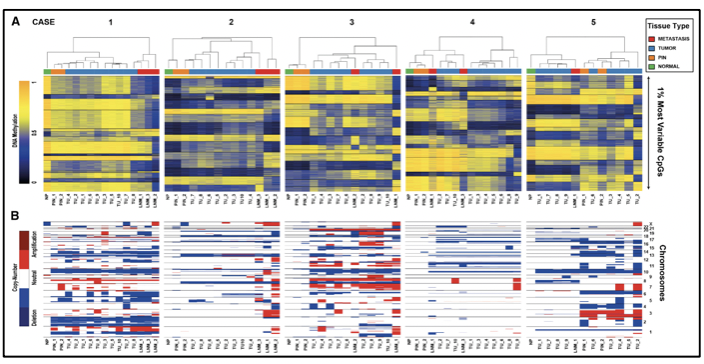
Molecular studies such as next-generation gene sequencing, as well as studies of circulating tumor cells, epigenetic studies, and microarrays, among others, provide us with evidence of a clonal expansion of prostate cancer with the different mutational events that occur in its genome. The somatic level at various stages of disease progression. There are investigations of the genomic composition of multiple sites of metastasis in the same patient or have followed the clonal evolution longitudinally in the tissues. To understand the origin of prostate cancer, its behavior over time, and the risks of disease progression, it is necessary to know the spatial and temporal genomic evolution of prostate cancer.
The clonal architecture of primary and metastatic tumors, allows us to know the clonal evolution during the progression of the disease (13). Various variants, as well as altered molecular pathways in both the primary tumor and metastasis, have been identified, confirming intratumor heterogeneity and thus evidencing that metastasis and resistance to androgen deprivation treatment occur due to different molecular alterations acquired over time (13).
Studies reveal that the loss or variant of the TP53 gene, as well as the loss of PTEN, occur before or at the beginning of metastasis, indicating that they are drivers of metastatic spread (13). In addition to the 7 molecular subtypes already mentioned (ETS fusions, variants in FOXA1, FLI1, SPOP, and IDH1) there may be an acquisition of new variants that lead to metastases, which could not be preferentially present in any of these subtypes (13).
Other important findings such as the androgen receptor (AR), which is altered in more than 60% of cases of metastatic prostate cancer, and which is also a mediator for resistance to androgen deprivation therapy (ADT), which can acquire new variants after metastasis (14). What is not yet clearly known is whether these rare subclones originated in the primary tumor or early in the metastases, possess alterations in the AR that subsequently promote resistance to ADT, or whether these alterations arise after metastasis and initial ADT treatment (13).
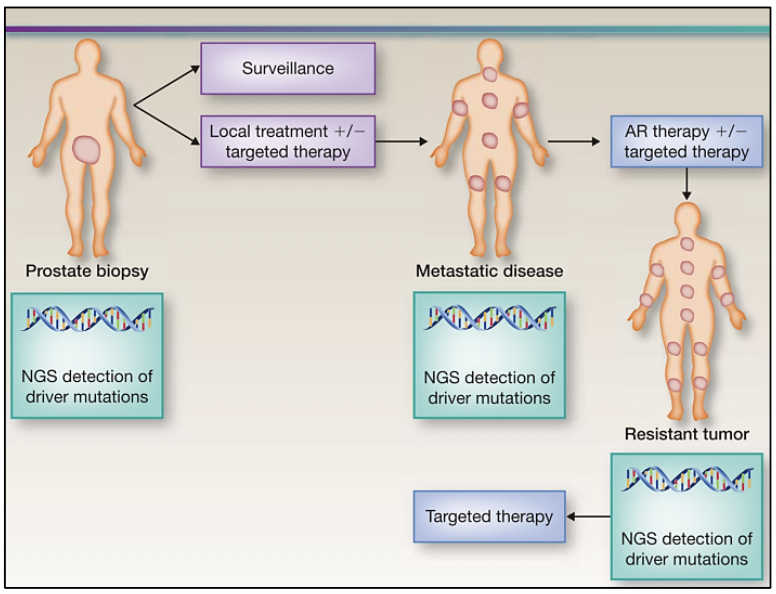
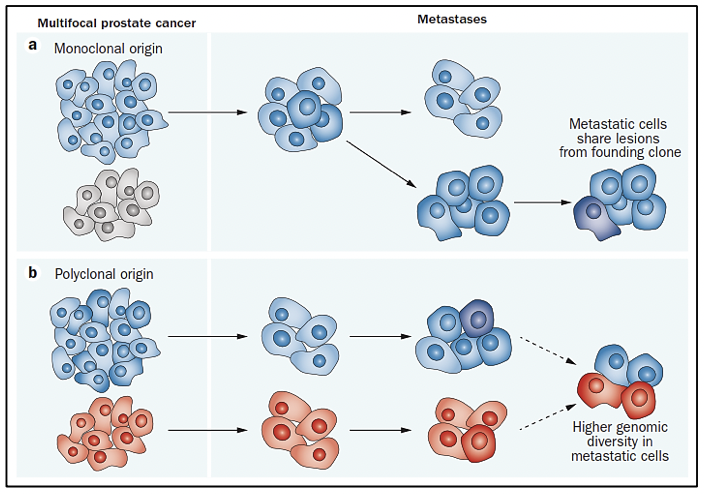
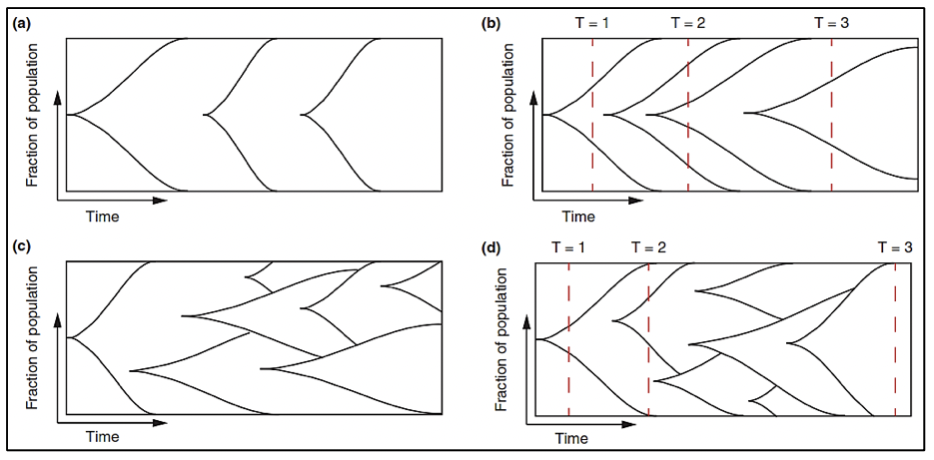
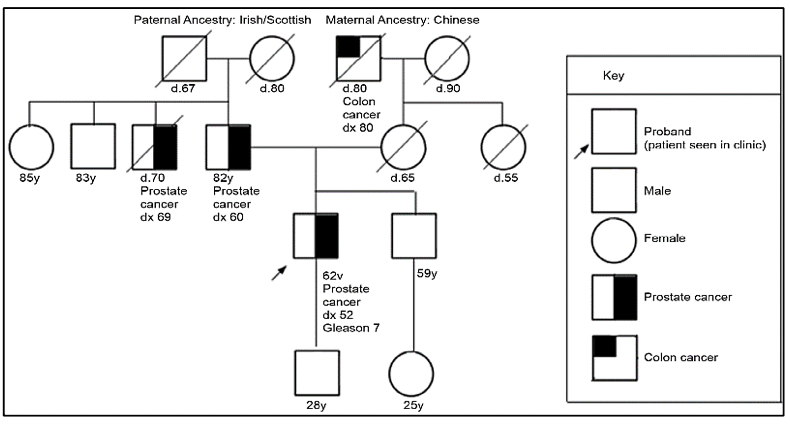
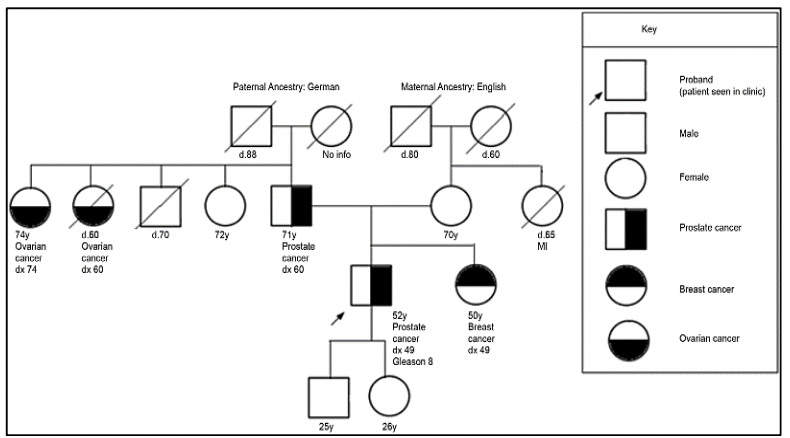
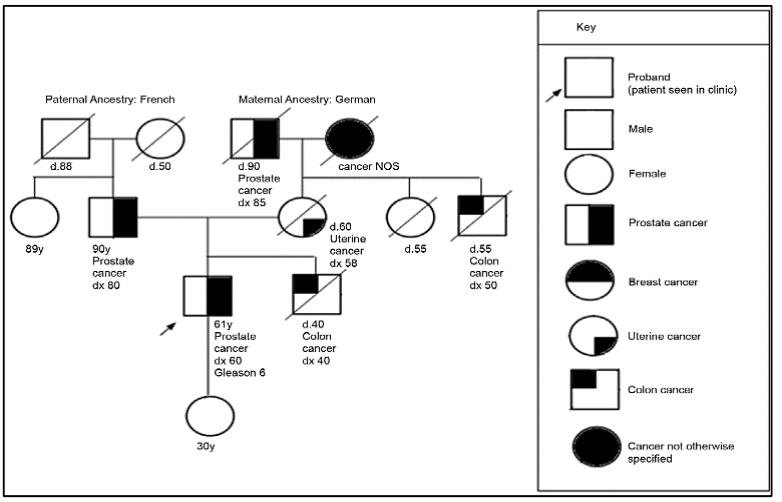
The study of the prostate cancer genome not only allows us to have a vision over time of the genomic alterations that occur during the different stages of prostate cancer but also to learn about the mechanisms of metastasis. Metastatic spread can occur through monoclonal or polyclonal seeding between metastases, or in waves originating from the primary tumor (14). It has also been shown that clonal propagation is not only unidirectional, for example, when metastatic subclones reach the surgical bed of the resected primary tumor (figure 1 y figure 2).
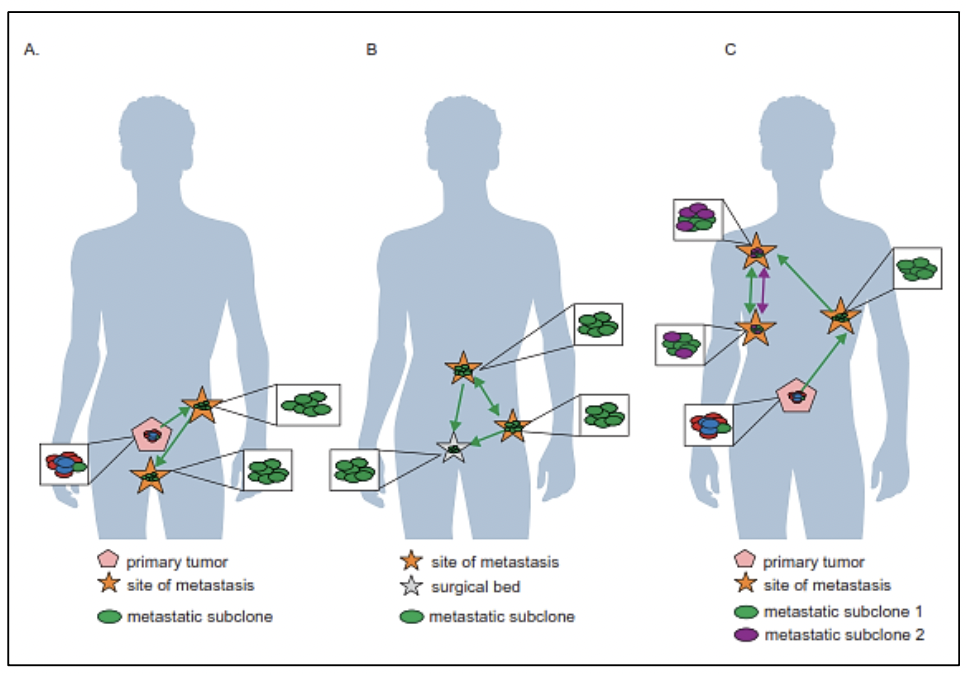
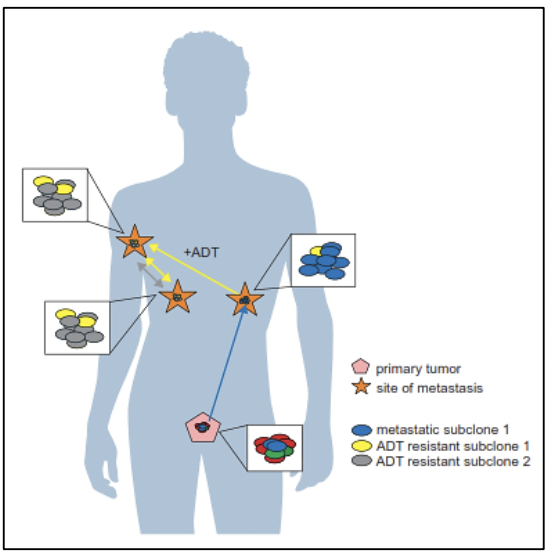
There is evidence that after radical prostatectomy, tumor samples have multiple intraprostatic cancer foci that are separate and may be biologically variable in their potential for aggressiveness and disease progression (15). Heterogeneity can be of two types, intratumor (different molecular alterations in multiple cancer foci in the tumor specimen from the same patient) and intertumor (such as different molecular alterations in patients with the same Gleason score) (15). Through next-generation gene sequencing, it has been possible to identify a variety of molecular alterations in prostate cancer such as gene fusions, amplifications, hemizygous and homozygous deletions, and/or point variants, which shows that there is genomic heterogeneity.
Prostate cancer originates through a clonal evolutionary process, due to a sequential accumulation of variants to the point where these variants promote the development of a neoplastic phenotype (these variants are called "drivers") (16), differs in its molecular composition at each stage of the disease. Some of these driver variants accelerate the acquisition of new variants by hindering the activity of some cellular mechanisms to detect or repair DNA damage or to respond with programmed cell death. The increase in the rate of variants leads to the acquisition of variants that have no immediate functional relevance for the cell (passenger variants) but that will later become relevant or even vulnerable as therapeutic targets (17-19).
To perform precision medicine in patients with prostate cancer, it is necessary that, at the time of diagnosis through biopsy, molecular analysis is requested to detect conductive variants in the tumor, and based on its molecular profile, determine monitoring and treatment. Subsequently, if the disease progresses or if there is resistance to treatment, it is also necessary to carry out a molecular analysis, because the molecular profile is variable in each stage of the disease.
BIOMARKERS IN PROSTATE CANCER
Biomarkers are widely used for screening, detection, and prognosis of prostate cancer, revolutionizing the diagnosis and monitoring of the disease. Despite various studies on prostate cancer, there is no molecular biomarker with a potential clinical application for diagnosis and stratification in all stages of the disease, from localized to metastatic. Prostate-specific antigen (PSA) is usually used routinely as a marker, however, it has a low positive predictive value in localized prostate cancer and does not differentiate the stage of the disease (20-21).
PCA3 (9q21–22) is a highly expressed non-coding mRNA in prostate tumors, which can be detected in urine and prostate fluid in patients with prostate cancer, in addition to being approved by the FDA and being available in various laboratories in the world. The cut-off point approved by the FDA is 25 since it is at this point that there is a balance between the sensitivity and specificity of the test (22). Several studies also use the cut-off point 35 (22). A PCA3 value > 35 in urine has a sensitivity of 66% and specificity of 76% for the diagnosis of prostate cancer compared to PSA in serum (specificity 47% and sensitivity 65%) (23). The study of PCA3 in urine obtained after the prostate massage is superior to serum PSA in predicting the result of the biopsy with a sensitivity and specificity of 70% and 80% respectively, it also has a negative predictive value of 90% (23). The urine test can predict the presence of prostate cancer in a biopsy, with PCA3 levels being independent of prostate volume and serum PSA (23). Elevated levels of PCA3 have even been shown in patients with elevated PSA and negative biopsies, which could help reduce the number of unnecessary biopsies (23).
In addition to being a common rearrangement in prostate cancer, the TMPRSS2-ERG gene fusion serves as a molecular biomarker in prostate cancer with a specificity of 90% and a positive predictive value of 94% (24). With the detection of this gene fusion, the probability of finding cancer in the biopsy increases from 15% to 90%, and even if the gene fusion is detected in urine and the biopsy does not detect cancer, it is necessary to repeat the biopsy, due to the high specificity of this biomarker (24). In addition, this gene fusion is associated with a poor prognosis in patients who carry it in the tumor (23). The combination of detection of TMPRSS2-ERG and PCA3 in urine improves the performance of PSA alone for prostate cancer detection and clinically meaningful cancer prediction (25).
Genetic alterations at the germinal level are molecular biomarkers, that allow the calculation of risk in patients with prostate cancer because it has been shown to influence the aggressiveness of prostate cancer and patient survival (23). In addition, the presence of variants in some of these genes is associated with the earlier presentation of cancer as well as a family history of prostate cancer.
Other promising biomarkers in prostate cancer are changes in DNA methylation, histone acetylation, or microRNAs, which can lead to gene silencing, resulting in altered gene expression without altering the DNA sequence (23). Hypermethylation of the glutathione S-transferase P1 (GSTP1) gene is one of the most prevalent (90%) and can be detected in both serum and urine of patients; however, it is not specific for prostate cancer since it has been seen in approximately 70% of cases of high-grade intraepithelial neoplasia (23).
EPIGENETIC ALTERATIONS AND METHYLATION STUDIES IN PROSTATE CANCER
Changes in DNA methylation together with epigenetic silencing of some genes are the earliest somatic changes identified in the development of prostate cancer (26). The GSTP1 gene encodes an enzyme responsible for protecting the cell from genome damage (6). The loss of GSTP1 expression is an early event in the onset of carcinogenesis since GSTP1 gene methylation has been shown in 5-10% of cases of inflammatory proliferative atrophy and in more than 70% of prostatic intraepithelial neoplasia. high grade (6). GSTP1 promoter methylation is associated with a risk of recurrence in patients with prostate cancer, which makes it a marker of recurrence (6).
CIRCULATING TUMOR CELLS IN PROSTATE CANCER
Among the new molecular biomarkers used in prostate cancer is the detection of circulating tumor cells (CTCs) in peripheral blood, and it is used as a prognostic tool since it has been proposed that the spread of CTCs in the blood is an essential mechanism of metastasis (21). Screening for CTCs is FDA-approved for monitoring the treatment of metastatic prostate cancer (21). In metastatic prostate cancer, the CTCs threshold per 7.5 ml of venous blood has been significantly determined as a prognostic marker for overall survival (21).
GENETIC PREDISPOSITION TO PROSTATE CANCER
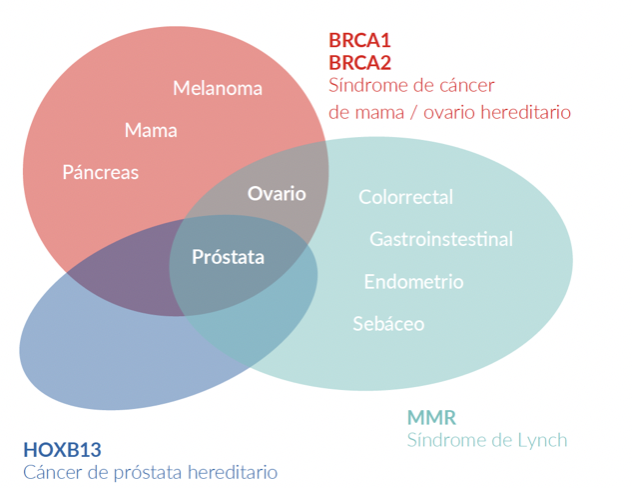
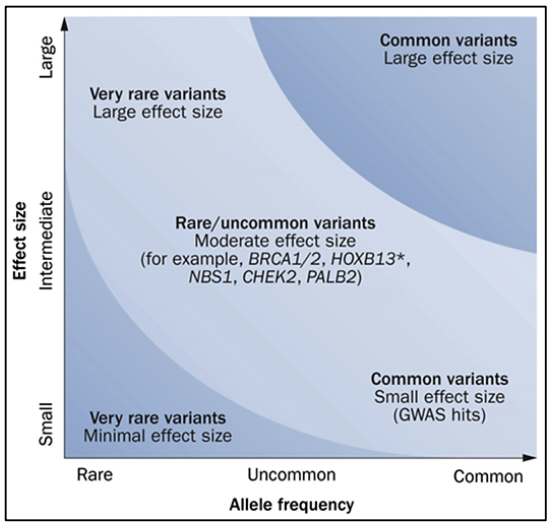
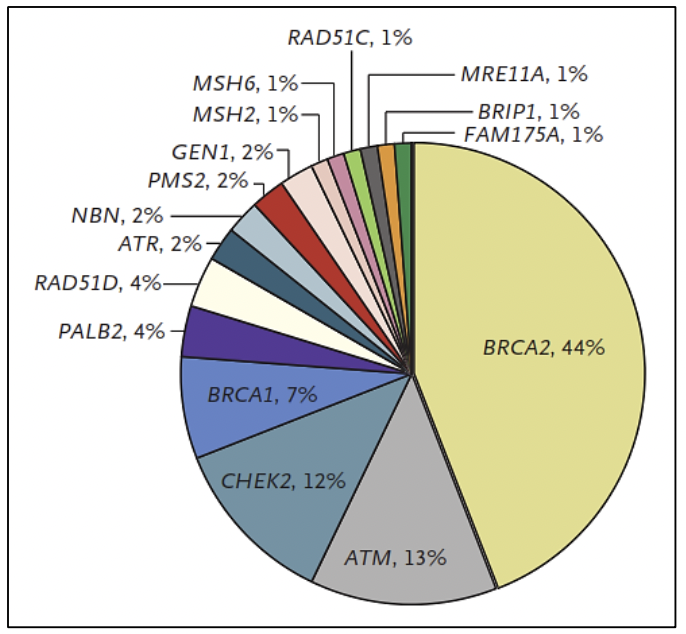
Prostate cancer has a hereditary component, which is characterized by the presence of cancer at an early age, having a family history of prostate cancer and other tumors, due to germline variants in BRCA1/2 genes, MMR (MLH1, MSH2, MSH6, PMS2), HOXB13, among others (27).
Reference criteria for genetic evaluation of patients with prostate cancer (27):
• Age less than or equal to 65 years.
• Gleason > 7 and family history of neoplasms related to Hereditary Breast Ovarian Cancer Syndrome.
• Family history of any cancer related to Hereditary Breast Ovarian Cancer Syndrome, hereditary prostate cancer, or Lynch syndrome, mainly in a first or second-degree relative.
For the evaluation of patients with a suspected genetic predisposition to prostate cancer, it is necessary to carry out a family tree, to be able to identify if we are dealing with a family at risk. According to the types of neoplasms, age of presentation, and inheritance pattern, we will be able to define the genetic syndrome and thus direct the molecular study to identify germinal variants in the patient and their relatives.
The HOXB13 gene has been identified as a prostate cancer susceptibility gene (28). Germline variants in the HOXB13 gene have been associated with a 2- to 8-fold increase in risk, these cases being those corresponding to hereditary prostate cancer (27). A germline variant in HOXB13, which is recurrent, G84E, p.(Gly84Glu), c.251G>A, has been frequently reported in patients diagnosed with prostate cancer at an early age (2.2%) and family history (3.1%) (28).
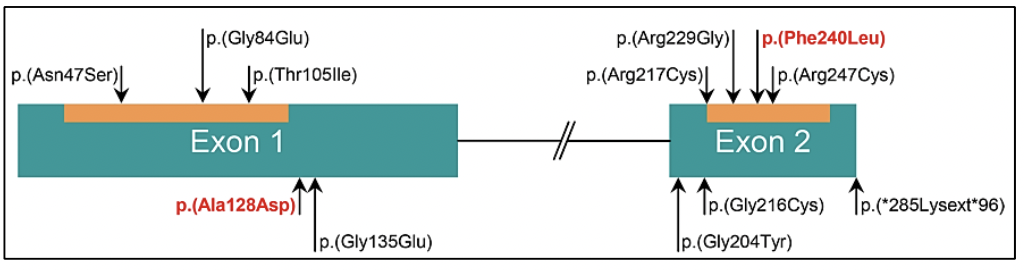
Germline variants in the BRCA1 and BRCA2 genes correspond to cases of Hereditary Ovarian Breast Cancer Syndrome, where in addition to the risk of breast cancer, ovarian cancer, melanoma, and pancreas, there is a risk in men to develop prostate cancer (27). Germline variants in the BRCA2 gene have been associated with a more aggressive phenotype of the disease (29). Likewise, germline variants in the BRCA2 gene confer a 7.3-8.6 times greater risk of developing prostate cancer before the age of 65, compared to non-variant carriers (28). In young patients, the prevalence of germline variants in the BRCA2 gene is 2.9%, conferring a 23-fold greater risk of developing prostate cancer up to 56 years of age (28).
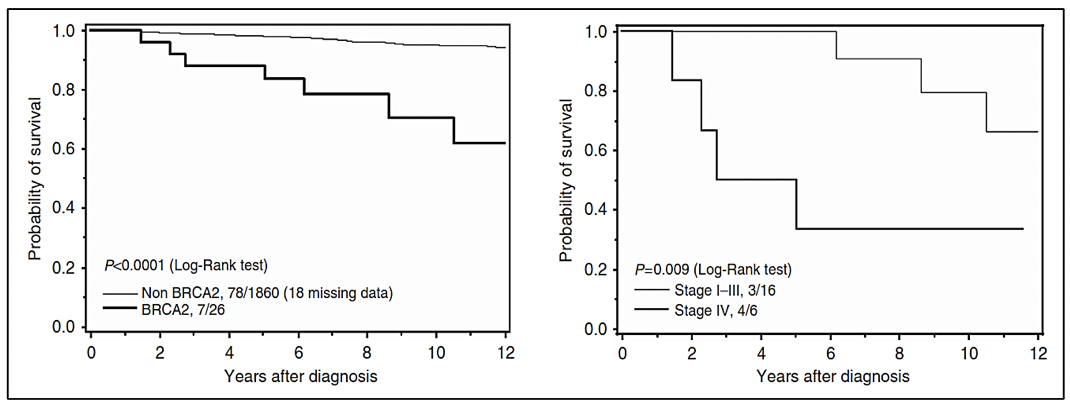
In cases associated with Lynch syndrome, due to variants in the MMR genes involved in the repair of DNA replication errors, the risk of prostate cancer is 3 times higher compared to the general population (27). In addition, germline variants have been reported in other DNA repair genes such as ATM, CHEK2, PALB2, RAD51D, and RAD51C, among others (30).
CONCLUSION
Knowing the molecular alterations of prostate cancer, as well as its molecular classification, genomic heterogeneity, and clonal evolution allows adequate management of patients. In addition, the collection of data from the patient's family history makes it possible to suspect a genetic predisposition to prostate cancer and thus refer him for timely genetic counseling and to be able to provide him with follow-up and therapeutic options.
Authorship contributions: The author participated in the genesis of the idea, project design, data collection and interpretation, as well as preparation of the manuscript for this research work.
Funding sources: Self-financed.
Conflicts of interest: The author declares no conflicts of interest in the publication of this article.
Received: May 15, 2022
Approved: July 06, 2022
Correspondence: Maria del Carmen Castro Mujica.
Address: 5440 Alfredo Benavides Avenue, Santiago de Surco, Lima-Perú.
Telephone number: 987597107
E-mail: mc.castro.mujica@gmail.com
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
REFERENCES