ARTICULO ORIGINAL
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2022 - Universidad Ricardo Palma10.25176/RFMH.v22i4.5094
CLINICAL-EPIDEMIOLOGICAL AND TREATMENT CHARACTERISTICS OF CHILDREN WITH COVID-19 IN A TERTIARY REFERRAL CENTER IN PERÚ
CARACTERÍSTICAS CLÍNICAS DE NIÑOS CON COVID-19 ADMITIDOS EN UN CENTRO TERCIARIO DE REFERENCIA EN EL PERÚ
Christian Chiara Chilet1, Medalit Luna Vilchez1, Julio Maquera-Afaray1,2, Blanca Salazar Mesones1, Diana Portillo Alvarez1, Ramiro Priale Miranda1, Franklin Mendoza1, Aldo Munayco1, Mitsi Santiago3, Jose W. López1,4
1Unidad de Atención Integral Especializada, Instituto Nacional de Salud del Niño San Borja, Lima, Perú.
2Facultad de Ciencias de la Salud, Universidad Privada de Tacna, Tacna, Perú.
3Coordinación técnica de epidemiología, Instituto Nacional de Salud del Niño San Borja, Lima, Perú.
4Facultad de Ciencias de la Salud, Universidad Científica del Sur, Lima, Perú.
ABSTRACT
Introduction:The COVID-19 pandemic has a great impact on children's health. This study describes the characteristics of hospitalized children at the Instituto Nacional de Salud del Niño San Borja (INSN-SB) in Perú. Methods:This was a retrospective study of patients with a confirmed diagnosis of COVID-19 from March to July 2020. Demographic, clinical, laboratory, radiological, and treatment information were collected. Data analysis included descriptive statistics and bivariate analysis to determine differences between patients in general wards and the Pediatric Intensive Care Unit (PICU). Results: We included 91 patients, 33 being females (36.3%). The most affected age group was children > 2 years of age (63 cases) with a median age of 6 years (IQR 3-10), and 61.5% were from Lima. The previous contact was determined in 30.8% of cases. A positive SARS CoV-2 PCR result was obtained in 50.6%. The presence of comorbidity was 53.8%. The most frequent symptoms were: fever (39.6%), general malaise (23.1%), cough (19.8%), and respiratory distress (14.3%). The presence of multisystem inflammatory syndrome in children (MIS-C) was confirmed in 6 patients. Antibiotics were administered in 76.9%. The most frequent radiological pattern was bilateral interstitial infiltrates (57.7%). Mortality was higher in patients in the ICU than in the hospitalization ward (27.3% vs. 4.3%, respectively; p = 0.020) Conclusions: COVID-19 in children presents mild and moderate clinical manifestations. The presence of comorbidity is an important factor for hospitalization, and mortality is high upon admission to critical care units.
Keywords: Child; Comorbidity; Coronavirus Infections; Mortality. (fuente: MeSH NLM).
RESUMEN
Introducción: La pandemia por COVID-19 representa un gran impacto en salud infantil, en este estudio se describe el comportamiento de esta enfermedad en pacientes pediátricos hospitalizados en el Instituto Nacional de Salud del Niño San Borja (INSN-SB) en el Perú. Métodos: Estudio retrospectivo de pacientes con diagnóstico confirmado de COVID-19 durante marzo a julio de 2020. Se recolectó información demográfica, clínica, laboratorial, radiológica y de tratamiento, para el análisis de datos se incluyó estadística descriptiva y un análisis bivariado para determinar las diferencias de pacientes en salas de hospitalización y la Unidad de cuidados intensivos pediátrico (UCIP). Resultados: Se incluyeron 91 pacientes, 33 de sexo femenino (36,3%). El grupo etario más afectado fueron los niños > de 2 años de edad (63 casos) con una mediana de edad de 6 años (RIC 1-8). 61,5% de pacientes procedían de Lima. El resultado de PCR SARS CoV-2 fue positivo en el 50,6%. La presencia de comorbilidad fue 53,8%. Los síntomas más frecuentes fueron fiebre (39,6%), malestar general (23,1%), tos (19.8%) y dificultad respiratoria (14,3%). La presencia de síndrome inflamatorio multisistémico (MIS-C) se confirmó en 6 pacientes. El uso de antibióticos representó 76,9%. El patrón radiológico más frecuente fue intersticial bilateral (57,7%). La mortalidad fue mayor en pacientes de UCI frente a los de salas de hospitalización (27,3% vs. 4,3%, respectivamente; p = 0.020). Conclusiones: El COVID-19 en niños presenta manifestaciones clínicas leves y moderadas. La presencia de comorbilidades es un factor importante de hospitalización, y la mortalidad es alta en pacientes admitidos a UCIP.
Palabras Clave: Niños; Comorbilidad; Infecciones por Coronavirus; Mortalidad. (fuente: DeCS BIREME).
INTRODUCTION
The coronavirus pandemic is the biggest public health crisis the world still has to face. It has been estimated that more than 90% of the cases correspond to the adult population and only between 1 to 5% occur in the pediatric population (1), of which more than 90% are asymptomatic, mild, or moderate forms of presentation, and only 5.9 % severe cases (2), this percentage is much lower compared to the 18.5% of severe cases reported in the adult population (3).
The association between the presence of comorbidity and severe disease has been reported in a study of 48 pediatric patients admitted to the PICU, of which 40 children (83%) had some comorbidity (4), in another study that included 651 children, 46% I present at least one comorbidity (5).
The development of MIS-C has been described, which can be severe and present significant cardiac involvement (6). Patients with MIS-C were found to have a 5-fold increased risk of being admitted to the PICU (5). In Peru, the incidence of COVID-19 in adults will continue to be 13 times higher than in children (7), however, the behavior of COVID-19 in pediatric patients with complex comorbidities has not been described.
The aim of this study is to describe the clinical, epidemiological, and treatment characteristics of children diagnosed with COVID-19 hospitalized at the INSN-SB, which is characterized as a highly complex specialized surgical center.
Methods
Design and study area.
During the period from March 1 to July 31, 2020, a retrospective qualitative and quantitative observational study was carried out
Population and sample:
For non-occasionally demonstrated convenience, all hospitalized patients under 18 years of age with a laboratory-confirmed diagnosis of COVID-19 who had the variables of interest were included (N=91). Consult the medical records of patients treated with COVID-19, the INSN-SB is a reference pediatric center with 306 beds. For the best care of patients with COVID-19, 24 beds were provided for patients with a confirmed case of COVID-19, 25 beds for patients with suspected COVID-19, and 12 beds for pediatric patients with PICU requirements.
Eligibility criteria: Patients with incomplete data were excluded and the clinical evaluation was performed by the pediatrician according to institutional care guidelines.
The definition of COVID-19 cases was considered according to the institutional standard of the Peruvian Ministry of Health (MINSA) (8), as well as the definition of the MIS-C of the World Health Organization (WHO), which includes children and adolescents from 0 to 19 years old with fever greater than or equal to three days and other criteria (9).
COVID-19 infection was diagnosed by the immunochromatographic blood test or by the polymerase chain reaction test of a nasopharyngeal swab, depending on their availability.
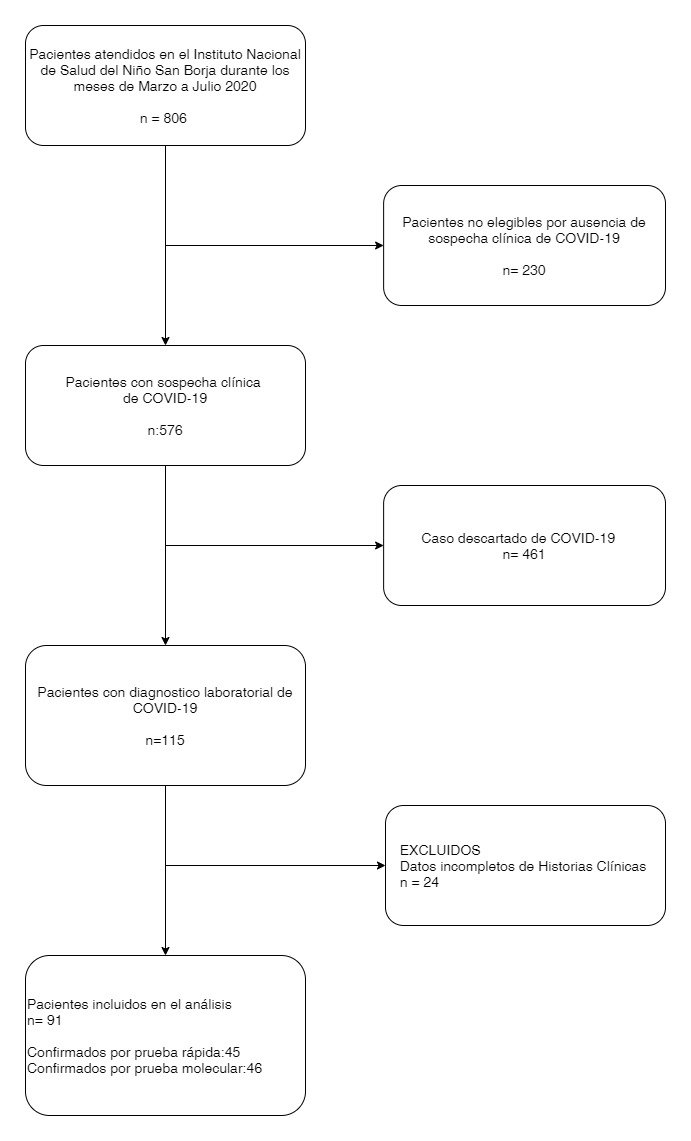
In addition, as part of the hospitalization criteria, confirmatory laboratory tests were performed on asymptomatic patients prior to the start of chemotherapy or in the preoperative period, the tests and images were indicated by the pediatrician and were performed according to institutional guidelines. (Figure 1)
Study variables
The epidemiological variables evaluated were sex, age, origin by department, weight, height, comorbidity and direct contact with COVID-19 patients. The clinical variables included were: date of onset of symptoms and the presence or absence of symptoms such as malaise, cough, shortness of breath, diarrhea, abdominal pain, nausea, vomiting, headache, runny nose, and pharyngeal pain. The clinical signs considered were fever, conjunctival injection, rash, dyspnea, snoring, wheezing, crackles, sub-crackles, seizures, pharyngeal exudate, pretibial edema or scaling of extremities, and raspberry tongue.
Procedures:
Laboratory tests for SARS CoV-2 infection were both rapid and molecular tests. In addition, the following were included: hemoglobin, leukocytes, leukocyte differential, platelets, albumin, creatinine, ferritin, C-reactive protein, procalcitonin, sedimentation rate (ESR), lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST) , total bilirubin, alkaline phosphatase, gamma-glutamyl transferase (GGT) prothrombin time (PT), D-dimer
Treatment received during COVID-19 infection is described based on drug groups (antibiotics, antifungals, antivirals, antiparasitics, anticoagulants, antipyretics, human immunoglobulin, corticosteroids, and vasoactive drugs)
Finally, the severity of the clinical picture, PICU requirement and admission date were evaluated, as well as the presence or absence of MIS-C; as well as, the hospital stays considering the final condition of the patient and the cut-off point as the last day of July 2020.
Statistical analysis.
The study data was collected and managed using the Integrated Hospital Management System (SIS Galen Plus) (10) and the REDCap electronic data capture tools (11,12) hosted at the INSN-SB.
A descriptive analysis was performed for the variables of interest; for quantitative variables due to an asymmetric distribution, medians and interquartile ranges (IQR) were analyzed, and for qualitative variables, frequency tables were analyzed.
The bivariate analysis was performed to identify the differences between the types of hospitalization (general wards and PICU), for qualitative variables the chi-square test and Fisher's test were used, while non-parametric tests were performed for numerical variables. Statistical analysis was performed with version 16 of the STATA program (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). All differences were considered with a value of p < 0.05.
Ethical aspects
This study was approved by the Institutional Research Ethics Committee of the INSN-SB with project code PI-429.
RESULTS
Between March and July 2020, 576 patients were admitted to the INSN-SB with suspected COVID-19, of which 19.9% (n=115) had a diagnosis confirmed by molecular or rapid testing, a total of 91 were analyzed patients. The majority of patients were male, with the predominant age group being schoolchildren: 61.5% (n=56) of the patients came from the city of Lima and 89.1% (n=81) had incomplete vaccination, 100% from who (n=22) were admitted to the PICU. (Table 1)
Table 1. Demographic characteristics of patients with COVID-19 seen at INSN SB (n=91).
| Characteristics | Total | ||
|---|---|---|---|
| n | % | ||
| Gender | |||
| Female | 33 | 36,3 | |
| Male | 58 | 63,7 | |
| Age (years) | 4 (1-8) | ||
| Source | |||
| Lima | 56 | 61,5 | |
| Provinces | 35 | 38,5 | |
| Incomplete vaccination | 81 | 89,1 | |
| Surgical admission | |||
| Emergency | 21 | 53,8 | |
| Elective | 18 | 46,2 | |
| Contact | |||
| Family | 24 | 26,4 | |
| Patient | 4 | 4.4 | |
| Unknown | 63 | 69.2 | |
| Diagnostic test | |||
| Quick test | 45 | 49,4 | |
| Molecular test | 46 | 50,6 | |
| X-ray | |||
| Normal | 45 | 63,4 | |
| Abnormal | 26 | 36,6 | |
| Presence of comorbidity | |||
| Yes | 49 | 53,8 | |
| No | 42 | 46,2 | |
| Presence of MIS-C | |||
| Yes | 6 | 6.6 | |
| No | 85 | 93.4 | |
| Patient's final condition | |||
| Live | 82 | 90.1 | |
| Deceased | 9 | 9.9 | |
Regarding comorbidity, they occurred in 53.8% (n=49), of which 68.8% (n=15) were admitted to the ICU. In 31.2% (n=15) the most frequent comorbidity was neurological and 24.4% (n=12) cardiological, followed by 14.2% (n=7) hematological and 14.2% (n=7) oncological, finally in patients admitted to PICU, the most frequent comorbidity was cardiological 40% (n = 6) and neurological 20% (n = 3). (Table 2)
Table 2. Comorbidities of patients with COVID-19 seen at INSN-SB (n=91).
| Comorbidities | Total | ||
|---|---|---|---|
| N | % | ||
| Presence of comorbidity | |||
| Yes | 49 | 53,8 | |
| No | 42 | 46,2 | |
| Type of comorbidty | |||
| Neurological | 15 | 31,2 | |
| Cardiological | 12 | 25,5 | |
| Hematology | 7 | 14,9 | |
| Oncology | 7 | 14,9 | |
| Gastrointestinal | 6 | 12,2 | |
| Trauma | 4 | 7,7 | |
| Pneumology | 4 | 7,7 | |
| Burn | 3 | 5,77 | |
| Other | 2 | 3,8 | |
The symptoms reported were general malaise 23.1% (n=21), cough 19.8% (n=18), respiratory distress 14.3% (n=13), vomiting 12.1% (n=11); as evidenced signs fever 39.6% (n=36), dyspnea 13.2% (n=12), snoring 9.9% (n=9) and seizures 5.5% (n=5). (Table 3)
In general, the majority of patients had a normal chest X-ray 63.6% (n=14), with differences evidenced between children admitted to hospitalization wards and those admitted to PICU (74% vs 38%, respectively) or radiological pattern abnormal (26% vs 61.9%, respectively) (p < 0.01). In addition, the most frequent radiological pattern was bilateral interstitial infiltrates in 57.7% (n = 15). (Table 3)
Table 3. Clincal and radiological characteristics of patients with COVID-19 seen at INSN-SB (n=91).
| Characteristics | Total | ||
|---|---|---|---|
| n | % | ||
| Symptoms | |||
| General malaise | 21 | 23,1 | |
| Cough | 18 | 19,8 | |
| Respiratory distress | 13 | 14,3 | |
| Vomiting | 11 | 11,2 | |
| Abdominal pain | 10 | 11,0 | |
| Rhinorrhea | 8 | 8,8 | |
| Nausea | 6 | 6,6 | |
| Diarrhea | 3 | 3,3 | |
| Pharyngeal pain | 3 | 3,3 | |
| Seizures | 2 | 2,2 | |
| Headache | 2 | 2,2 | |
| Signs | |||
| Fever | 36 | 39,6 | |
| Dyspnea | 12 | 13,2 | |
| Snoring | 9 | 9,9 | |
| Sibilants | 5 | 5,5 | |
| Seizures | 5 | 5,5 | |
| Subcrepitant | 4 | 4,4 | |
| Adenopathies | 4 | 4,4 | |
| Rash | 3 | 3,3 | |
| Pretibial edema | 2 | 2,2 | |
| Raspberry tongue | 1 | 1,1 | |
| Conjunctival injection | 1 | 1,1 | |
| Chest radiological pattern | |||
| Bilateral interstitial | 15 | 57,7 | |
| Unilateral Alveolar | 8 | 30,7 | |
| Unilateral interstitial | 3 | 11,5 | |
| Bilateral alveolar | 2 | 7,6 | |
Regarding mortality, differences were observed between children admitted to hospitalization wards and those admitted to PICU (4.3% vs 27.3%; p < 0.02). (Table 4).
Table 4. Mortality reported in patients in hospital wards and PICU with COVID-19 attended at INSN-SB (n=22).
| Characteristics | Hospitalization rooms | PICU | Total | p value | |
|---|---|---|---|---|---|
| Survival | n=69 | n=22 | n=91 | ||
| Yes | 66 (95,6%) | 16 (72,7%) | 82 (90,1%) | ||
| No | 3 (4,3%) | 6 (27,3%) | 9 (9%) | 0.061 | |
In relation to MIS-C, only 6.6% (n = 6) of the patients presented the criteria established by the WHO, most of these patients did not require PICU admission (n = 4).
In the patients who had laboratory tests on admission, we found that the main laboratory alteration was the levels of C-Reactive Protein (CRP) 9.4 mg/L (2.1 – 96.7) and D-Dimer 0.69 ug/ ml(0.27 - 1.65), we did not show greater alteration with respect to procalcitonin 0.14 (0.05-0.65) or in the values of TGP, AST and creatinine, with respect to the blood count the median of the hemoglobin was 11.8 g/dl (9.8 - 12.8), in addition we did not find leukocytosis, neutrophilia, left shift or marked lymphocytopenia (leukocytes at 10800x103/uL(7430 - 1450), lymphocytes 3040x103 /uL (1820-4910), neutrophils 5045x 103/uL (3095-9240) By IgM serology, Mycoplasma pneumoniae was the most isolated microorganism 9.9% (n=9), followed by Cytomegalovirus 3 .3% (n=3), Epstein Barr 2.2% (n=2) and Influenza 1.1% (n=1); gram-positive germs were isolated in 42.9% (n=9), in a smaller proportion were isolated Gram negative bacteria 38.1% (n=8) and fungi 19% (n=4) (Table 5).
Table 5. Laboratorial and microbiological characteristics of patients with COVID-19 seen at INSN-SB.
| Characteristics | Medium | Interquartile range | |
|---|---|---|---|
| Hematology | |||
| Hemoglobin | 11,8 | 9,8-12,8 | |
| Leukocytes | 10800 | 7430-14570 | |
| Lymphocytes | 3040 | 1820-4910 | |
| Monocytes | 630 | 450-960 | |
| Neutrophils | 5045 | 3095-9240 | |
| Rods | 0 | 0,0 | |
| Platelets | 314000 | 233000-399500 | |
| Biochemistry | |||
| C-reactive protein | 9,4 | 2,1-96,7 | |
| ESR | 28 | 15-50 | |
| Creatinine | 0,33 | 0,22-0,45 | |
| Albumin | 4,01 | 3,49-4,5 | |
| AST | 31 | 22-43 | |
| GPT | 17 | 13-32 | |
| Ferritin | 276,10 | 93,8-468,1 | |
| Total Bilirubin | 0,62 | 0,34-1,22 | |
| Prothrombin time | 14,8 | 13,7-16,2 | |
| Fibrinogen | 344,5 | 266-502 | |
| D-dimer | 0,69 | 0,27-1,65 | |
| Procalcitonin | 0,14 | 0,05-0,65 | |
| CPK | 110 | 40-254 | |
| CPK-MB | 29.9 | 24,5-38 | |
| Lactate | 2 | 1,4-3,4 | |
| LDH | 650 | 442-848 | |
| Microbiology | n | % | |
| Mycoplasma | 9 | 9,9 | |
| Cytomegalovirus | 3 | 3,3 | |
| Epstein Barr | 2 | 2,2 | |
| Influenza B | 1 | 1,1 | |
| Herpes 1 | 1 | 1,1 | |
| Parvovirus B19 | 1 | 1,1 | |
| Type of insulation | n=21 | % | |
| Gram positive | 9 | 42,9 | |
| Gram negative | 8 | 38,1 | |
| Fungi | 4 | 19,0 | |
Regarding the drugs administered, 76.9% (n=70) of the patients received antibiotics, of which the ones with the highest prescription were cephalosporins in 58.6% (n=41) and glycopeptides in 40% ( n=28), it is also important to mention that 40% (n=25) patients received carbapenems and macrolides such as azithromycin were used in 17.1% (n=12). Antiparasitic were administered in 25.3% (n=23) of the patients, hydroxychloroquine in 17.4% (n=4). Antipyretics, mainly paracetamol, were used in 89% (n=81) patients, while 18.7 (n=17) received anticoagulants, 31.9% (n=29) corticosteroids, and 28.6 (n=26) vasoactive drugs. Regarding MIS-C, 5.5% (n=5) of patients received human immunoglobulin (Table 6).
Table 6. Frequency of drug prescription in patients with COVID-19 seen at INSN SB (n=91).
| Medicines | Total | ||
|---|---|---|---|
| n | % | ||
| Antibiotics | 70 | 76,9 | |
| Cephalosporins | 41 | 58,57 | |
| Glycopeptides | 28 | 40 | |
| Carbapenems | 25 | 35,71 | |
| Penicillins + Beta lactamase inhibitors | 14 | 20 | |
| Macrolides | 12 | 17,14 | |
| Aminoglycosides | 9 | 12,86 | |
| Lincosamides | 9 | 12,86 | |
| Penicillins | 8 | 11,43 | |
| Sulfonamides | 7 | 10 | |
| Quinolones | 3 | 4,29 | |
| Oxazolidinones | 1 | 1,43 | |
| Nitrofurones | 1 | 1,43 | |
| Antifungals | 8 | 8,8 | |
| Azoles | 7 | 87,5 | |
| Echinocandins | 1 | 12,5 | |
| Antivirals | 1 | 1,1 | |
| Nucleotide analogues (Acyclovir) | 1 | 100 | |
| Antiparasitics | 23 | 25,3 | |
| Nitroimidazoles | 20 | 86,96 | |
| 4 amino-quinolines (Hydroxychloroquine) | 4 | 17,39 | |
| Benzimidazoles | 1 | 4,35 | |
| Anticoagulants | 17 | 18,7 | |
| Low molecular weight heparin (Enoxaparin) | 12 | 71 | |
Discussion
In this study, it was found that most of the patients presented a mild to moderate clinical picture, almost a third of the patients required admission to the PICU in the context of associated comorbidities. In addition, a group of patients with diagnostic criteria for MIS-C is described, all of whom evolved favorably.
Similar to what was reported in our study, according to the CDC, the median age of 576 hospitalized children was eight years (13), with a predominance of males (13,14). In addition, only a third of our patients had family contact (26.4%), unlike what was reported in other studies where they indicate a history of family contact in more than 40% of cases (14, 15), this finding can be explained due to the lack of information about the diagnosis in the family environment.
Although in our study respiratory symptoms and fever were the most frequent clinical manifestations, 33% of patients presented gastrointestinal symptoms, in the literature the presentation of gastrointestinal manifestations is variable and is currently described in more than 20% of cases (6,13,16). Similar to other studies, the most frequent coinfection was Mycoplasma pneumoniae and to a lesser extent with other respiratory viruses (14,17,18). In this regard, it has been described that respiratory viral infections could predispose the development of super aggregated bacterial infections such as M. pneumoniae, due to altered respiratory mucociliary clearance and immune system response (19); however, M. pneumoniae infection could also precede viral infection (20).
Similar to what we reported, it was found that the most frequent comorbidities were neurological in 11% (65/614) and hematological-oncological in 8% (45/614) (6). However, another study found comorbidity more asthma (12.3%), followed by sickle cell anemia (7%) (21), many of these comorbidities are related to congenital pathologies, unlike in adults. According to severity, in our study it was reported that 68.2% of patients in the PICU presented comorbidities, being a high percentage as in another study that describes more than 80% of patients hospitalized in the PICU with comorbidities (4)
We observed an elevation of CRP and D-dimer, in accordance with what was found in the literature, a variety of laboratory alterations have been described in COVID-19, especially serum inflammatory markers (6,15,22,23), One study found levels elevated CRP, procalcitonin, interleukin-6, ferritin, and D-dimer, in relation to severity (22). Our findings may be due to the higher proportion of patients with mild and moderate symptoms and because only the initial tests are included in the analysis.
In relation to the cases of MIS-C, in our study we found six patients with a diagnosis of MIS-C and two required PICU admission similar to that described in another Peruvian report (⅛) (23), while in another study they found that MIS-C cases are 5 times more likely to be admitted to the PICU, however, no patient with MIS-C died (6). In both studies, all progressed favorably. In contrast, a systematic review of MIS-C in children and adolescents reported that MIS-C appears to be a more severe condition with 68% (531/783) of cases requiring PICU admission (24).
Antibiotic prescription was high despite having few microbiological findings. In the literature, a meta-analysis describes both in adults and children that 71.3% had a prescription for antibiotics, despite the fact that the prevalence of bacterial coinfections and COVID-19 was low and the frequency of secondary bacterial infection in patients was 14 ,3% (26), these results suggest that antibiotics should be prescribed under a clear suspicion of bacterial infection in patients with COVID-19.
In our study, the use of hydroxychloroquine was rarely used and the use of immunoglobulin was reserved mainly for patients with MIS-C criteria, while in a systematic review 7.8% of children received hydroxychloroquine, 4.1% corticosteroids and 3 .1% immunoglobulin (15) Regarding hydroxychloroquine, the findings can be explained by the little scientific evidence initially reported on it, unlike intravenous immunoglobulin, which is accepted as immunomodulatory therapy in the management of Kawasaki disease and in the MIS-C (6,23,26,27).
Our study has some limitations, such as presenting retrospective observational information, lack of complete baseline tests for COVID-19, partly explained by the diversity of clinical criteria for the request. Regarding the diagnosis of MIS-C, not all patients underwent a serological test for SARS-CoV 2, and only those with a positive confirmatory test were included in the present study.
Conclusions
In conclusion, SARS CoV-2 infection in children presents mild and moderate clinical manifestations. Finally, a group of patients presented comorbidities, this being greater in those admitted to the PICU and in those who died.
Authorship contributions: C.Ch., M.L, J.M., B.S., D.P., R.P., F.M., A.M, and J.W.L planned the study. C.Ch., M.L, J.M., B.S., D.P., R.P., J.B., M.S. and J.W.L collected the data. C.Ch., J.W.L and M.L. performed the statistical analysis and the generation of figures. All authors reviewed the manuscript.
Funding sources: self-financing.
Conflicts of interest: The authors declare no conflict of interest.
Received: July 1, 2022
Approved: August 19, 2022
Correspondence: Christian Manuel Chiara Chilet.
Address: Av. Agustin de la Rosa Toro 1399 - San Borja.
Telephone number: (511) 230 0600
E-mail: cchiarach@insnsb.gob.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
REFERENCES