ORIGINAL ARTICLE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2023 - Universidad Ricardo Palma10.25176/RFMH.v23i2.5637
SPERM DNA INTEGRITY AND REPRODUCTIVE TOXICITY OF LEAD NITRATE IN ADULTS MALES MICE
INTEGRIDAD DEL ADN ESPERMÁTICO Y LA TOXICIDAD REPRODUCTIVA DEL NITRATO DE PLOMO EN RATONES MACHOS ADULTOS
Jonathan H. Vásquez Cavero
 1, 2a,
José M. Gonzáles Daga
1, 2a,
José M. Gonzáles Daga
 1,2b,
1,2b,
Rosmary Y. López Sam
 1,2b,
Pilar V. Pino Velásquez
1,2b,
Pilar V. Pino Velásquez
 1c
1c
,
José L. Pino Gaviño
 1,3a,
Betty Shiga Oshige
1,3a,
Betty Shiga Oshige
 1,3a
1,3a
1Laboratory of Reproduction and Developmental Biology, Departamento de Zoología. Instituto de
Investigación en Ciencias
Biológicas “Antonio Raimondi” (ICBAR). Universidad Nacional Mayor de San Marcos (UNMSM). Lima, Peru.
2Animal Reproduction Physiology Laboratory. Instituto de Investigación en Ciencias Biológicas
“Antonio Raimondi”,
Facultad de Ciencias Biológicas, UNMSM. Lima, Peru.
3Centro de Investigación de Recursos Naturales (CIRNA) Universidad Nacional Mayor de San Marcos
(UNMSM). Lima, Peru.
aBiologist.
bMaster in Molecular Biology.
cMaster in Assisted Reproduction Procedures.
ABSTRACT
Introduction: Lead toxicity has been linked to different diseases in humans and several evidences suggest a strong relationship with the observed damage on reproductive function in humans and rodents. Methods: Mice were given a single dose of lead nitrate (NP) (50mg/kg/bw), which were euthanized seven days post-injection with the aim of evaluating sperm to come out from the seminiferous tubules and are in transit through the epididymis. Also, the TUNEL test was done to evaluate the sperm DNA fragmentation. Results: The decrease in body weight in mice treated with LN (p < 0.05), suggest a toxic systemic effect. However, the same didn’t happen on the reproductive system because the weights of the testes, epididymis, prostate and seminal vesicles were not altered (p > 0.05), in the same way physiological values such as sperm concentration and motility didn´t decrease with the treatment (p > 0.05). Transit and sperm maturation in the epididymis would not be affected by the LN, and because we did not observe increased sperm DNA fragmentation in the treated group (p > 0.05), sperm protamination would be fulfilling its protective role on murine genetic material avoiding genotoxic damage by LN. Conclusion: The intraperitoneal administration of 50mg/kg/pc of LN for seven days does not cause systemic toxicity or effect on spermatogenesis in mice.
Keywords: Sperm concentration, DNA fragmentation, sperm motility, lead nitrate, sperm protamination. (Source: MeSH-NLM)
RESUMEN
Introducción: La toxicidad del plomo se ha relacionado a diferentes patologías en humanos y varias evidencias sugieren una fuerte relación con el daño observado sobre la función reproductiva en humanos y roedores. Método: Se proporcionó a ratones una dosis única de nitrato de plomo (NP) (50mg/kg/pc), los cuales fueron eutanizados siete días postinyección con el objetivo de evaluar los espermatozoides que han salido de los túbulos seminíferos y están en tránsito por el epidídimo; además, se evaluó la fragmentación del ADN espermático mediante el ensayo TUNEL. Resultados: La disminución del peso corporal en ratones, tratados con NP (p < 0,05), sugiere un efecto tóxico sistémico. Sin embargo, no sucedió lo mismo sobre el sistema reproductivo, debido a que los pesos de los testículos, epidídimos, próstata y vesícula seminal no fueron alterados (p > 0,05); de igual manera, los valores fisiológicos como conteo y movilidad espermática no disminuyeron con el tratamiento (p > 0,05). El tránsito y maduración de los espermatozoides en el epidídimo no sería afectado por el NP, y al no observar aumento en la fragmentación del ADN espermático en el grupo tratado (p > 0,05), la protaminación espermática estaría cumpliendo su rol protector sobre el material genético murino, por lo que no hubo daños genotóxicos por el NP. Conclusiones: La administración intraperitoneal de 50mg/kg/pc de NP, por siete días, no causa toxicidad sistémica ni efecto en la espermatogénesis en ratón.
Palabras Clave: Concentración espermática, fragmentación del ADN, movilidad espermática, nitrato de plomo, protaminación espermática. (Fuente: DeCS–BIREME)
INTRODUCTION
The general population is exposed to metals at low concentrations voluntarily (food supplements) or
involuntarily
through ingesting contaminated food or water and contact with contaminated soil, dust and air (1).
Lead is a contaminant widely distributed among heavy metals in all environmental and biological systems
(2).
The detection and prevention of lead toxicity is an important public health problem (3).
Although the population's exposure to lead has decreased in the last three decades with the introduction
of low-lead
paints, lead-free petroleum, and the ban on lead solder in food cans (4),
Subclinical exposure remains in other sources such as lead pipes, toys, insecticides, oil refineries,
construction
material, firearm bullets, X-rays, and industrial by-products (3).
We must add the high exposure of the populations that live near the mines, tailings, and deposits of
this metal, such as
in Oroya and Callao, where high levels of lead in the blood of the inhabitants have been reported,
especially children (5)
and even in newborns (6), so its potential risk to human health is still a
subject of much debate.
Lead toxicity has been related to different pathologies in humans (7), and its
relationship with the observed damage to reproductive function in humans and rodents (8,9) is suggested.
Most reports indicate that workers exposed to Pb+2 exhibit decreased sperm density and a high rate of
teratozoospermia (10).
Lead occurs in nature as an oxide or salt; thus, lead acetate (AP) has been shown to produce infertility
in mice (11,12);
evaluation from the first to fifth-week post-injection of AP at a single dose of 100 mg/kg body weight
(bw), increased
oxidative stress and sperm morphological abnormalities, while decreased tail epididymal sperm count
(13).
In turn, lead nitrate (Pb(NO3)2) (NP) has a multitude of uses and is a product of
economic importance (14),
it is embryotoxic in rats (15) but not teratogenic (16) and its genotoxic effects are still highly controversial (17,18).
It has been reported that 44.8 mg/kg/pc of NP induces DNA damage in blood cells even after seven days of
exposure (14). The mechanism by which lead exerts toxic effects on the
reproductive system has not yet been fully elucidated. Still, it
has been reported to induce oxidative stress in different tissues with the consequent generation of
reactive oxygen
species (ROS) (13,19).
Damage from ROS leads to infertility by two mechanisms: damage to the sperm membrane that decreases
sperm motility and
its ability to fuse with the oocyte, and damage to sperm DNA that alters the paternal genomic
contribution to the
embryo. Sperm DNA fragmentation has been related to many reproductive aspects (20) and is predictive of fertilization and pregnancy rates (21) demonstrating its evaluation's importance. Currently, there are no
reports on the effect of NP on the integrity of sperm
DNA. Considering that exposure to lead is a serious public health problem, this work attempts to clarify
whether a
single dose of NP (50mg/kg/pc ) affects male reproductive characteristics and sperm DNA integrity
assessed seven days
post-injection.
METHODS
Design and study area
Preclinical experimental study of cases and controls, area of experimental biology.
Population and sample
The sample consisted of male mice (N = 20) (Mus musculus) 6-7 weeks old, of the Balb C strain; the case group (GT) (experimental) was made up of 10 mice who were administered intraperitoneally (ip) a single dose of Lead Nitrate (50 mg/kg/pc), the Control Group (CG) was made up of 10 mice who received bidistilled water by the same route. All were kept in vivarium conditions: temperature from 25ºC to 27ºC, with free access to balanced food (Purina-Peru), water ad libitum and a photoperiod of 14/10 light/dark hours.
Variables
Independent variables: Sex (male, treatment male mice).
Dependent variable: Reproductive quality of the male mouse. A data collection sheet was made with
all the variables of the research study
where the findings were recorded.
Procedures
1. Treatment: The mice were divided into two groups; The treated group (TG) (n = 10) was administered the NP seven days post-injection, the body weight was recorded and the animals were euthanized by cervical dislocation, recording the weight of the testicles, epididymis, prostate and seminal vesicle using an analytical balance with 0.001 g sensitivity. This post-injection evaluation time allows us to analyze the sperm after its transit through the epididymis (22). Sperm concentration and motility were recorded, sperm DNA fragmentation was evaluated by the TUNEL technique.
2. Obtaining sperm: The tail of the epididymis was dissected, washed with PBS (pH 7.4), and in 0.5ml of Flushing medium (MediCult®, Copenhagen, Denmark), several incisions were made allowing sperm to be released for 10 minutes at 37°C. , the sperm content was fully recovered in 1.5ml polypropylene tubes (Axygen Scientific).
3. Evaluation of sperm motility and concentration: A spermatozoon was considered to have Progressive Motility (PM) when it moved, Non-Progressive Motility (NPM) to those that did not move and only had one movement (23). Sperm count was performed in a Newbauer chamber.
4. Sperm DNA fragmentation: Sperm DNA fragmentation was evaluated using the TUNEL assay, which assesses the presence of free 3'-hydroxyl ends identified by the enzyme TdT (terminal deoxynucleotidyl transferase) that catalyzes the addition of fluorescently labeled deoxyuracil triphosphates in sperm cells. DNA strand breaks. The In Situ Cell Death (Roche, Perú)was used; Briefly, and following the supplier's instructions, the technique consists of fixing the spermatozoa with 4% paraformaldehyde, washing in PBS, permeabilizing the spermatozoa membrane, and incubating one hour with the TdT enzyme at 37°C in the dark. A positive control was included, incubated with 50 units of DNases for 30 minutes at 37°C before adding the enzyme, and a negative control with no enzyme was added. The visualization of the spermatozoa was carried out in a fluorescence microscope, the green color evidenced damage to the spermatic DNA. 250 cells were analyzed for each individual.
Statistic analysis
The results were evaluated using the statistical package SPSS 17.0 for Windows. The Shapiro-Wilk and Leneve test was performed to verify the normality and homoscedasticity of the data. Differences between groups were analyzed using the Student's t-test (weights, motility and sperm concentration) and the Mann Whitney U test (sperm DNA fragmentation). Results are expressed as mean ± SE (standard error), n = 10. A value of p < 0.05 was considered statistically significant.
Ethical aspects
The care and handling of the animals were carried out in accordance with the ethical guidelines of our institution and the National Research Council for the care and use of laboratory animals (24).
RESULTS
Significant differences were observed between the final and initial body weights during the experiment, being lower and negative in the NP Group (p < 0.05) (Table 1). The consequences of the reproductive organs: testicles, epididymis, prostate, seminal vesicle (Table 1), physiological values of mobility (Figure 1) and sperm concentration (Figure 2).
Table 1. The difference in body weight, weights of testis, epididymis, prostate, and seminal vesicle in the treatment group Pb(NO3)2 (50mg/kg/pc) vs Control (H2O). *p < 0.05, Student's test. Mean ± Standard error (SE), n = 10.
|
|
Control (H2O) |
Pb(NO3)2 (50mg/kg/pc) |
|---|---|---|
|
∆Body weight |
1.7752 ± 0.5694* |
0.1632 ± 0.0991 |
|
Testicle Weight |
0.0913 ± 0.0030 |
0.0932 ± 0.0037 |
|
Epididymal Weight |
0.0295 ± 0.0011 |
0.0302 ± 0.0016 |
|
Prostate Weight |
0.0122 ± 0.0012 |
0.0129 ± 0.0011 |
|
Seminal Vesicle Weight |
0.2591 ± 0.0291 |
0.2675 ± 0.0476 |
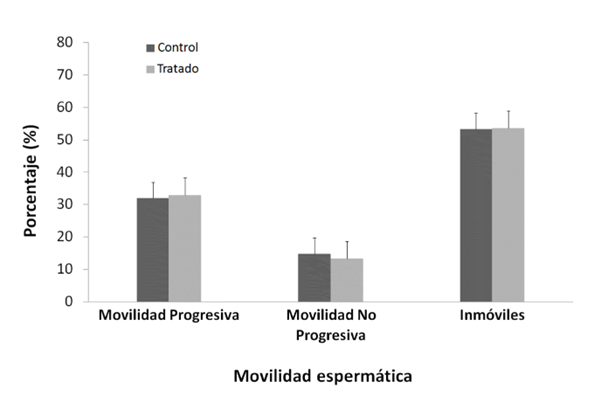
Student's test. Mean ± Standard error (SE), n=10.
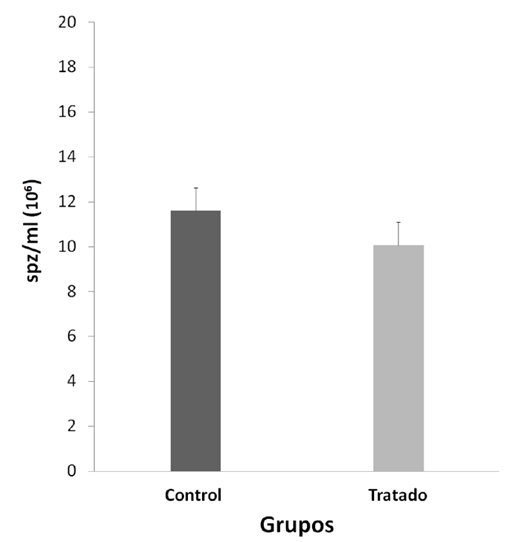
Student's test. Mean ± Standard error (SE), n=10.
Table 2. DNA fragmentation test (TUNEL) of mouse sperm cells treated with lead nitrate versus control.
|
|
Control (H2O) |
Pb(NO3)2 (50mg/kg/pc) |
|---|---|---|
|
DNA fragmentation |
0.2 |
0.8 |
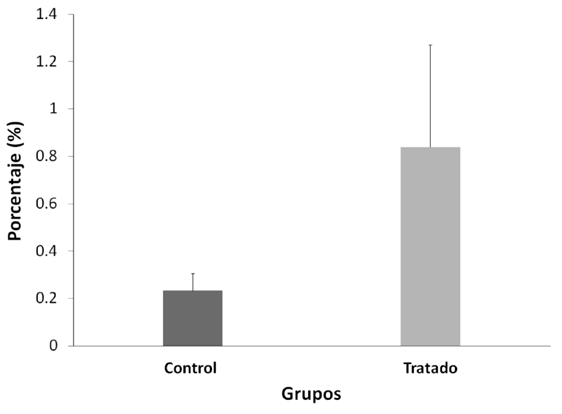
Mann-Whitney U-test. Mean ± Standard error (SE), n=10.
The sperm count evaluated with TUNEL between both groups was not altered after treatment with NP (p>0.05) (Figura 3); the Positive Control in this test was 99%, and the Negative Control 0%.
The DNA fragmentations did not show significant differences (p > 0.05).
DISCUSSION
The present work investigates the effects of a single dose of NP in mouse spermatozoa. We must note that a systemic
toxic effect of NP was evidenced by decreasing the body weight of the treated mice (Table 1, p < 0.05).
However, the weights of the testicles, epididymis, prostate, and seminal vesicle, which are androgen-dependent target
organs (25), did not decrease, so NP would not be exerting a toxic or anti-androgenic effect on the male reproductive system(Table 1, p > 0,05).
NP would not have a localized effect on sperm physiology because no significant differences were observed in sperm
motility and concentration between the two groups
(Figures 1 and Figura 2, p > 0.05); sperm transit and maturation in the epididymis would not be affected by NP. In this research, within the spermatogenic cycle of the mouse, we evaluated the spermatozoa deposited in the epididymiS (22), these spermatozoa have already exchanged their histones for protamines, which allows them to strongly condense the
paternal genome within the nucleus (26). While the histones of a somatic cell compact the DNA forming nucleosomes, protamines, proteins unique to spermatozoa,
wind and condense DNA into much larger subunits known as toroids (26). In this way, sperm chromatin experiences strong compaction, 6 times more than in a somatic cell (27) protecting the integrity of sperm DNA (25,27,28).
However, the multiple cysteine residues that surround the spermatic nucleus are oxidized to form disulfide bridges
between protamines and stabilize chromatin during the final stages of sperm maturation (28),
achieving complete protection of sperm DNA only in the epididymis itself. Devi
(14)observed DNA damage in blood cells in mice evaluated seven days after administration of a dose of NP (44.8 mg/kg/pc), a
similar concentration used in this work (50 mg/kg/pc). Therefore, we expected that NP causes damage to the DNA of the
spermatozoa; however, our results show the opposite (Figure 3, p > 0,05), which demonstrates the importance of protamination as a sperm protection mechanism against PN (29).
At the same time, we believe that the epididymis, having many antioxidant enzymes (30),
could adequately counteract the well-documented effect of lead on the induction of ROS in different tissues (31).
Furthermore, it has been shown that the effects of lead on the reproductive system depend on the exposure time,
returning to normal values after a prolonged exposure time (32),
which suggests that an animal exposed to this metal is capable of adapting to its toxic effects; In the present work, by
administering a single dose of NP, we ruled out the possibility of not observing damage to the reproductive system,
sperm physiology, and sperm DNA integrity due to a prolonged exposure time to NP.
As limitations of the study we had the logistical nature in the institution where the work was carried out; Mainly due
to power cuts during the weekends that harmed the dosage and the continuity of the monitoring of the animals. In
addition, the impossibility of entering the investigation pavilion on Sundays due to lack of surveillance personnel. All
this caused delays and repetition of the experimental design.
CONCLUSIONS
We conclude that a single dose of Lead Nitrate 50mg/kg/pc does not alter reproductive parameters or damage sperm DNA in mice.
Authorship contribution: The authors participated in the genesis of the idea, project
design, development, data collection and interpretation,
results analysis, and manuscript preparation.
Financing: Office of the Vice Chancellor for Research of the Universidad Nacional Mayor
de San Marcos (Project with monetary funds,
Code 091001301).
Interest conflict: The authors declare that they have no conflict of interest in the
publication of this article.
Recevied: November 21, 2022.
Aprobed: February 13, 2023.
Correspondence: José Luis Rafael Pino Gaviño.
Address: Av. German Amézaga 375, cercado Lima-Peru.
Phone +51 992169186
E-mail: jpinog@unmsm.edu.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES
