CLINICAL CASE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2023 - Universidad Ricardo Palma10.25176/RFMH.v23i2.5655
GASTROINTESTINAL DYSAUTONOMIA AS A MANIFESTATION OF
TRANSVERSE MYELITIS: CASE REPORT
DISAUTONOMÍA GASTROINTESTINAL COMO MANIFESTACIÓN DE MIELITIS TRANSVERSA: REPORTE DE CASO
Abel Lugo Peláez
 1,a,
Viridiana Robles Sánchez
1,a,
Viridiana Robles Sánchez
 1,a,
Rodrigo Esteban Sóstenes
1,a,
Rodrigo Esteban Sóstenes
 1,a,
Nancy Rosalía Bertado Ramírez
1,a,
Nancy Rosalía Bertado Ramírez
 1,b,
Arturo García Galicia
1,b,
Arturo García Galicia
 1,c,e,
Álvaro José Montiel Jarquín
1,c,e,
Álvaro José Montiel Jarquín
 1,d,e
1,d,e
1 Unidad Médica de alta Especialidad, Hospital de Especialidades de Puebla, Centro Médico Nacional,
General de División, Manuel Ávila Camacho, Instituto Mexicano del Seguro Social.
a Internal Medicine Resident.
b Medical specialist in Neurology.
c Medical specialist in Pediatric.
d Medical specialist in Gastroenterology.
e Master of Medical Sciences and Research.
ABSTRACT
Inflammatory transverse myelitis is a rare condition that affects one or more levels of the spinal cord. Its etiology includes multiple sclerosis, infectious causes, or disorders within the spectrum of neuromyelitis optica. It presents acutely with motor, sensory, and/or dysautonomic symptoms, such as those related to the gastrointestinal and urinary systems. Diagnosis is based on symptomatology, evolution, and is confirmed by lumbar puncture, magnetic resonance imaging, and complete blood analysis.
We present a clinical case of a patient with transverse myelitis who presented with gastrointestinal symptoms, motor symptoms, and was diagnosed with magnetic resonance imaging.
Keywords: Transverse myelitis, demyelinating, dysautonomia, gastrointestinal. (Source: MeSH – NLM).
RESUMEN
La mielitis transversa, de origen inflamatorio, es una afectación rara de la médula espinal que afecta a uno o varios niveles. La etiología incluye esclerosis múltiple, causas infecciosas o trastornos del espectro de la neuromielitis óptica. Se presenta de forma aguda, con síntomas motores, sensoriales y/o disautonómicos como los gastrointestinales y urinarios. El diagnóstico se basa en la sintomatología, evolución y se confirma por punción lumbar, resonancia magnética nuclear y analítica sanguínea completa.
Se presenta el caso clínico de una paciente con mielitis transversa, que debutó con sintomatología gastrointestinal, síntomas motores y confirmación diagnóstica con resonancia magnética nuclear.
Palabras Clave: Mielitis transversa, desmielinizante, disautonomía, gastrointestinal (Fuente: DeCS – BIREME).
INTRODUCTION
Transverse myelitis (TM) of inflammatory origin is an acute or subacute demyelinating disease, reflecting bilateral or segmental spinal cord injury, developing
without prior neurological disease, including motor, sensory, and/or autonomic dysfunction (1).
Etiology includes parainfectious, autoimmune, and demyelinating diseases (2).
Its incidence is from 1.34 to 4.6 per million inhabitants per year, regardless of gender, ethnicity, or geographic area. The clinical picture can evolve in hours
to days and present a unilateral or bilateral evolution. The diagnosis must demonstrate spinal cord inflammation and exclude other pathologies, based on
gadolinium-enhanced Nuclear Magnetic Resonance (NMR) and cerebrospinal fluid (CSF) (3).
Corticosteroids are the first line treatment; methylprednisolone is the choice in acute treatment and can be continued with plasmapheresis or immunoglobulin in case
of unsatisfactory response (4).
The case of a young patient with initial symptoms of digestive dysautonomia, which is a rare presentation, is presented.
CASE REPORT
A 26-year-old female with previous diagnoses of depressive disorder for five years without treatment, constipation with 10-year follow-up, managed with laxatives
and with poor response, hypercontractile esophagus subtype sphincter lower esophagus hypertensive of 9 months of evolution, with management with neuromodulators,
prokinetic and proton pump inhibitors, with poor response.
Two exploratory laparotomies were performed, the first three years ago due to colonic occlusion and the second one year ago due to small bowel occlusion.
Her current condition began with progressive esophageal dysphagia, heartburn, and epigastric pain. Subsequently, she presented paresis and paresthesia in the thoracic
limbs and progressed to quadriparesis in less than 1 month, with an episode of bilateral amaurosis lasting approximately 15 minutes with full recovery of vision. An
esophagram was performed, which did not show changes in esophageal contractility. NMR of the brain and cervical spine showed a 17-mm hyperintense lesion at C6-C7,
suggesting a demyelinating plaque. Lumbar puncture reports clear, colorless CSF, pH 8, leukocytes 2 cells/mm3, glucose 42 g/dL, total protein 0.2 mg/dL, chlorine 125
mmol/L, and culture without bacterial development. Hematic cytometry, blood chemistry, and serum electrolytes are reported normal. The viral panel was reported to be
non-reactive to hepatitis B and C viruses and human immunodeficiency virus. The electroencephalogram without evidence of epileptic activity, the immunological profile
is shown in Table 1.
|
Table 1: Immunological profile |
|
|---|---|
|
anti-nuclear antibody (ANA) (IFI) |
Positive 1:100 coarse speckled nuclea |
|
Anti-double-stranded DNA antibody (dsADN) |
Negative |
|
Anti-reagin antibody (V.D.R.L.) |
not reactive |
|
Antistreptolysin O antibody |
181 UI/ml |
|
Anti-Smooth Muscle Antibody |
Negative |
|
Anti-Scl-70 antibody (Anti-topoisomerase 1) |
Negative |
|
Anti-Smith antibody |
Negative |
|
Anti-La (SS-B) Antibody in Serum |
Negative |
|
Anti-Jo-1 antibody (histidyl-tRNA synthetase) |
Negative |
|
Anti-Mitochondrial Antibody (IFI) |
Negative |
On physical examination: ophthalmological assessment without alterations is reported; Daniels muscle strength 3/5 in thoracic limbs, 2/5 in pelvic limbs; muscle
stretch reflexes (REM) +/++++ in the right pelvic limb, and REM ++/++++ in the rest of the extremities. Bilateral indifferent plantar response and preserved
sensitivity.
Treatment was started with bolus methylprednisolone, 1gr intravenously every 24 hours for five days; after not showing improvement, human immunoglobulin G was
administered, total dose 0.4gr/kg of weight/dose for 5 days. He presented clinical improvement at one month of follow-up with partial recovery of muscle strength
3/5 on the Daniels scale in the lower extremities and a decrease in gastrointestinal symptoms.
Follow-up cervical, thoracic and lumbar NMR month later reported demyelinating plaque at C6-C7 characterized by hypointense spindle-shaped lesion on T1 and FLAIR,
and hyperintense on T2 without gadolinium enhancement (See Figure 1).
DISCUSSION
Inflammatory transverse myelitis is a neurological pathology diagnosed with increasing frequency and represents a diagnostic and therapeutic challenge. This report
illustrates a case of early transverse myelitis with digestive-type dysautonomic symptoms with constipation and esophageal dysphagia.
Centromedullary pathology is distinguished from brain lesions and peripheral neuropathies by a well-defined sensory level, below which temperature perception is altered
(4).
In the patient in the reported case, no thermosensory alterations were documented, probably because the diagnosis of centromedullary TM was not considered. Cerebrospinal
fluid analysis can rule out infectious causes and support the diagnosis of an inflammatory pattern with moderate pleocytosis, elevated proteins, no oligoclonal bands (OCB),
and, occasionally, an increased IgG index, (5), which is consistent with this case.
NMR confirms the presence of a demyelinating spinal cord lesion, identifying the different types of TM and differentiating them from other structural, vascular,
and neoplastic lesions (6).
The characteristic findings in adults are T2 hyperintense lesions that enhance with gadolinium in 50–90% of cases. It can be located centrally in multiple segments
of the cord, most frequently in the thoracic region (7).
In the reported case, the centromedullary lesion did not enhance with gadolinium and the extension was in cervical segments (6-7).
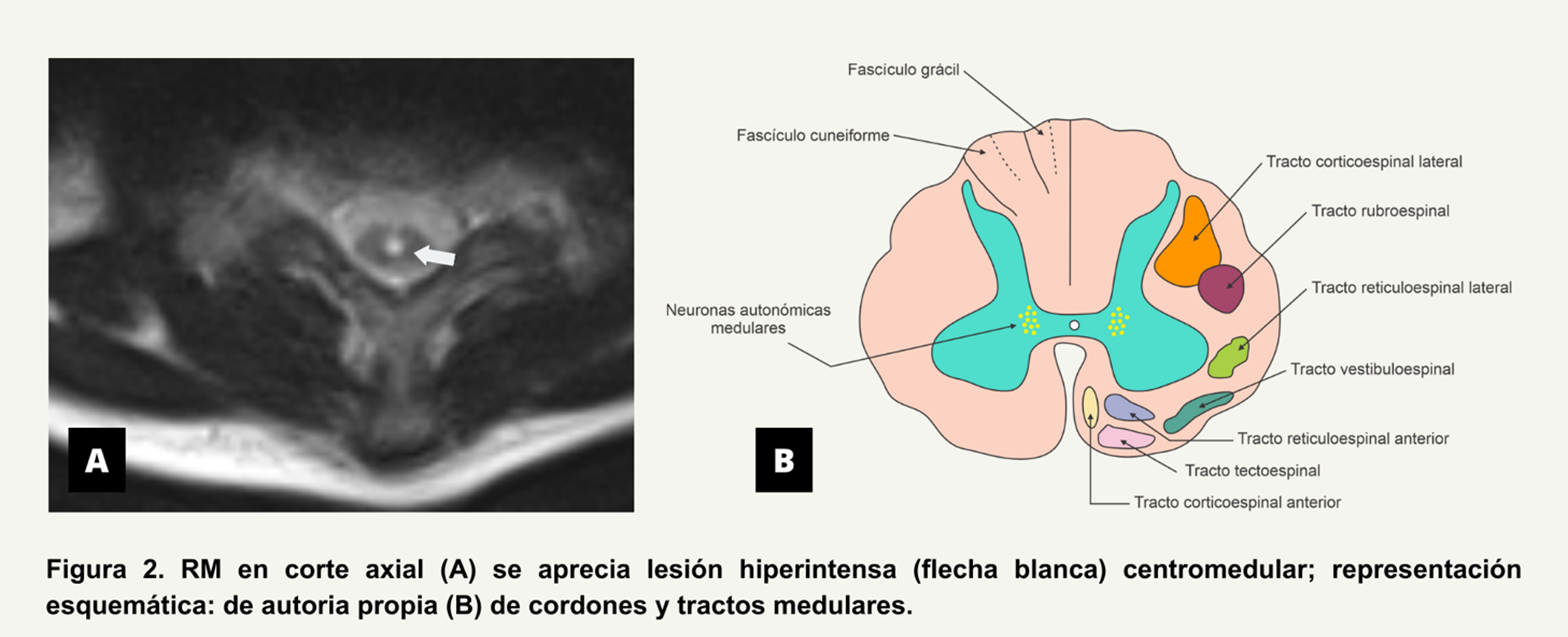
The main clinical manifestations in TM are motor, sensory, and/or autonomic, according to the site of involvement. Complications can be classified according to the stage of the disease. The acute phase is frequently characterized by acute urinary retention and constipation, and chronic complications include major depression, chronic or recurrent urinary tract infection, and paraplegic pressure ulcers. Intestinal dysfunction as in this case is the result of involvement of the parasympathetic, sympathetic, and motor nerves. From parasympathetic involvement, the Vagus nerve and the S2-4 nerves that are responsible for innervation of the foregut, midgut, and hindgut result in a slow transit time. In combination, slow transit and increased sphincter tone, along with loss of rectal fullness, can lead to severe constipation (8). In the present case, chronic constipation even conditioned two episodes of intestinal occlusion that required surgical management. This is a peculiar presentation as similar reports of intestinal occlusion, perforation, and necrosis are rare. The involvement of spinal cord autonomic neurons is noteworthy (Figure 2).
The goals of treatment in the acute phase are to halt progression and initiate resolution of inflammation and clinical recovery (9).
Corticosteroids are the first-line management, given intravenously in high doses. The second line is immunoglobulin or plasmapheresis, although its therapeutic effects are
still controversial (10).
The patient in this case received treatment with boluses of methylprednisolone without good clinical response, with subsequent rescue therapy with immunoglobulin. The
clinical improvement at discharge was minimal, and more noticeable at one month of follow-up. In similar cases, recovery with physical rehabilitation is described between
1 and 3 months, but it can be a long process and take up to 2 years. Lack of initial recovery in the first 3 to 6 months predicts complete recovery is unlikely (11).
The patient, in this case requires follow-up to assess autonomy, motor alterations, and dysautonomic manifestations, due to the level of involvement in C5-C7. The management
of chronic constipation should include hygienic-dietary measures, establishing an intestinal program that includes adequate fluids, adequate diet, activity and programmed bowel
movements, and the use of laxatives (1).
Authorship contribution:
The authors participated in the genesis of the idea, project design, development, data collection and interpretation,
results analysis, and manuscript preparation.
Financing:
Self-financed.
Interest conflict:
The authors declare that they have no conflict of interest in the publication of this article.
RECEIVED:
March 30, 2023.
APPROVED:
April 20, 2023.
Correspondence:
Nancy Rosalía Bertado Ramírez.
Address:
Calle 2 Norte, 2004, Colonia Centro , CP 72000 Puebla Pue.
Telephone :
222 438 9809
E-mail:
nancy.bertado@imss.gob.mx
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES