CLINICAL CASE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2023 - Universidad Ricardo Palma10.25176/RFMH.v23i2.5694
RECURRENT NEONATAL GASTRIC PERFORATION:
AN UNUSUAL CASE REPORT
PERFORACIÓN GÁSTRICA NEONATAL RECURRENTE: REPORTE DE UN CASO INUSUAL
VELARDE PAREDES, P.
 ,
CHUMBE MENDOZA, G.
,
CHUMBE MENDOZA, G.
 ,
,
VILCHEZ APONTE, J.

1Santa Rosa Hospital
2Universidad Continental
aPhysician Assistant Specialist in Pediatric Surgery
bMaster
ABSTRACT
Neonatal gastric perforation is a rare but potentially fatal pathology; its recurrence is even more infrequent, and there are few reports in the international literature. We present the case of a premature newborn (32 weeks) and low birth weight (1725g) who, on the first day of life, presented a surgically corrected gastric perforation and on the fourth postoperative day was re-operated with a presumed diagnosis of gastric raffia dehiscence, finding a new gastric perforation, which was surgically repaired. On both occasions, the surgical intervention was early. The subsequent evolution and outcome were favorable.
Keywords: Spontaneous Perforation, stomach, perforation, newborn. (Source: MeSH–NLM)
RESUMEN
La perforación gástrica neonatal es una patología rara pero potencialmente mortal; su recurrencia es aún más rara, son escasos los reportes en la literatura internacional. Se presenta el caso de una recién nacida prematura (32 semanas) y bajo peso al nacer (1725g) que el primer día de vida presentó una perforación gástrica corregida quirúrgicamente y al cuarto día postoperatorio es reintervenida con presunción diagnóstica de dehiscencia de rafía gástrica, hallándose una nueva perforación gástrica, la cual fue reparada quirúrgicamente. En ambas ocasiones la intervención quirúrgica fue temprana. La evolución posterior y desenlace fueron favorables.
Palabras clave: Perforation gástrica neonatal, neonato, perforación (Fuente: DeCS–BIREME)
INTRODUCTION
Neonatal gastric perforation is a rare condition that, if not diagnosed and treated promptly, can be potentially lethal. It has an annual incidence of 1 in 1800 admissions to a
neonatal intensive care unit, as reported by Yang et al in a study conducted at a tertiary-level pediatric hospital (1).
It represents 4% of gastrointestinal perforations in neonates (2).
Mortality can be as high as 60% (3),
but in recent years it has been reduced, with survival rates reaching 73% (4).
Although in certain cases it is possible to identify the causal agent of the perforation, such as congenital anomalies of the gastrointestinal tract (pyloric, duodenal, jejunoileal
atresia, esophageal atresia with tracheoesophageal fistula, intestinal malrotation), or the performance of procedures (positive pressure ventilation, endotracheal intubation, nasogastric
tube placement); in many cases there is no apparent cause for the perforation, and these cases are considered idiopathic or spontaneous. Neonatal gastric perforation has been associated
with certain risk factors such as prematurity, low birth weight, asphyxia/hypoxia, severe infections, and administration of certain medications (NSAIDs, corticosteroids)
(3,4).
The clinical presentation can include abdominal distension, vomiting, lethargy, bloody stools, fever, or hematemesis; abdominal X-ray may show pneumoperitoneum or intestinal obstruction
(5).
Surgical findings reveal that the perforation, although it can occur in any location of the gastric wall, most commonly occurs on the anterior surface adjacent to the greater curvature
(50% to 64% of cases) (2,4).
The management of this condition includes broad-spectrum antibiotic treatment, early surgical intervention, and intensive care.
Being neonatal gastric perforation an uncommon pathology, recurrence of the lesion is an exceptional situation, so few cases have been reported in international publications
(6-9).
This report aims to present an unusual case of a neonate with gastric perforation and successful surgical repair, who subsequently experienced recurrence in a different location,
which was repaired with satisfactory progress and favorable outcome.
CLINICAL CASE
The current case involves a newborn (NB) female infant, who is the product of a 28-year-old mother's first pregnancy, referred to the Hospital Santa Rosa of Pueblo Libre, a tertiary-level
hospital in Lima, Peru. The mother is from Tocache, a locality in the Peruvian jungle, and was diagnosed with severe preeclampsia and treated with magnesium sulfate and nifedipine. She
received prenatal care at the local health center in Tocache without any complications until the time of referral. The gestational age was determined to be 32 weeks based on the last
menstrual period and ultrasound. The mother underwent a cesarean section after pulmonary maturation (dexamethasone), with the result of a baby with a weight of 1.725 g, APGAR 7 to 1
minute, 8 to 5 minutes and heart rate of 140 beats per minute. Immediately after birth, the infant presented constant whimpering, respiratory distress, and low oxygen saturation, requiring
intubation and transfer to the neonatal intensive care unit (NICU) for mechanical ventilation with minimal parameters. She was extubated at 10 hours of life and was transferred to
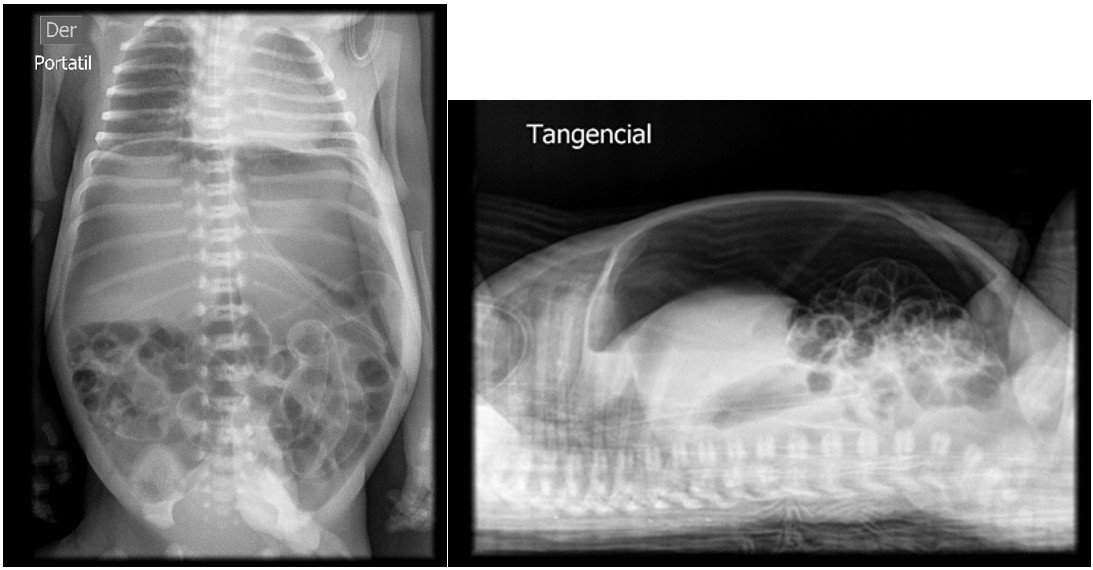
intermediate care, where she remained on gastric rest with an orogastric tube (OGT) for gravity feeding. The infant developed abdominal distension, prompting an abdominal X-ray at 20 hours
of life, which revealed pneumoperitoneum and absent bowel sounds (figure 1). Empirical antibiotic therapy with Meropenem and Vancomycin is initiated. The patient was evaluated by a pediatric surgeon,
and surgical intervention was scheduled with a presumptive diagnosis of intestinal perforation.
She underwent surgery at 9 hours after the diagnosis was made (at 29 hours of life), revealing a single gastric perforation measuring 3.5 cm in length at the lesser curvature on the anterior side, with free bilious intestinal secretion in the cavity. The abdominal cavity was approached through a right infraumbilical transverse laparotomy, and the perforation was sutured in two layers. The cavity and intestinal loops were irrigated with saline solution (0.9% NaCl). The patient responded well to the surgical procedure and was subsequently intubated and transferred to the neonatal intensive care unit (NICU) according to the protocol.
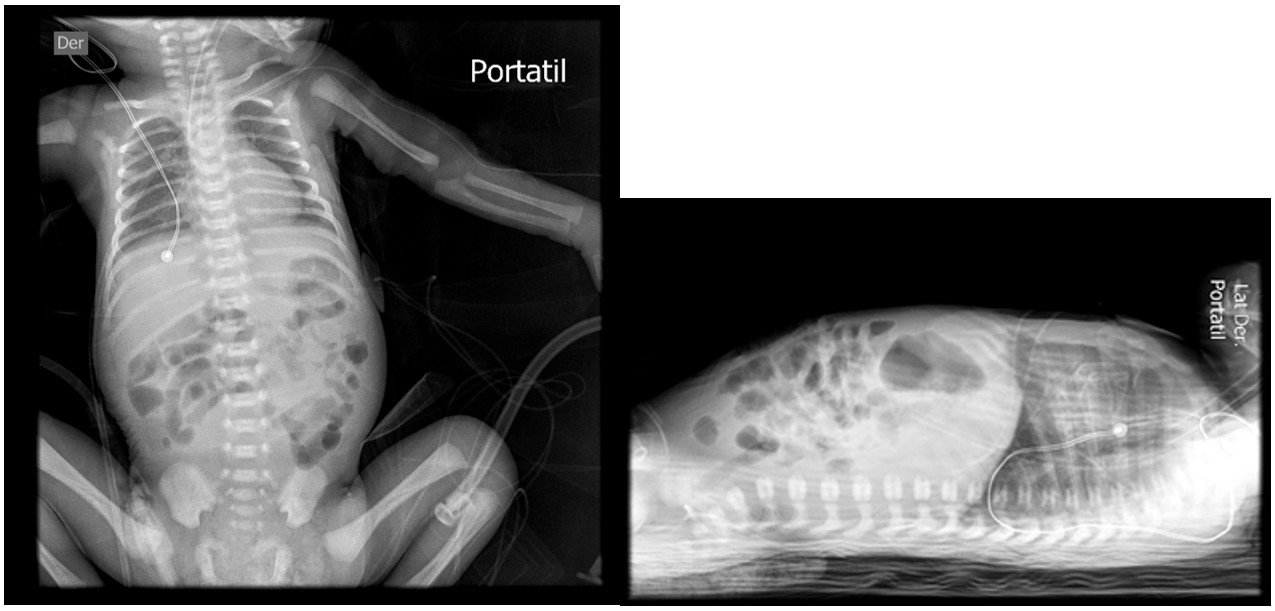
In the immediate postoperative period, the patient presented coagulation disorder, hyperglycemia, hyperchloremia, sustained hypoalbuminemia, and hypermagnesemia. Parenteral nutrition was initiated after the placement of a peripherally inserted central venous catheter. On the second postoperative day, an abdominal X-ray showed resolution of the pneumoperitoneum (figure 2). She experienced a 25% weight loss.
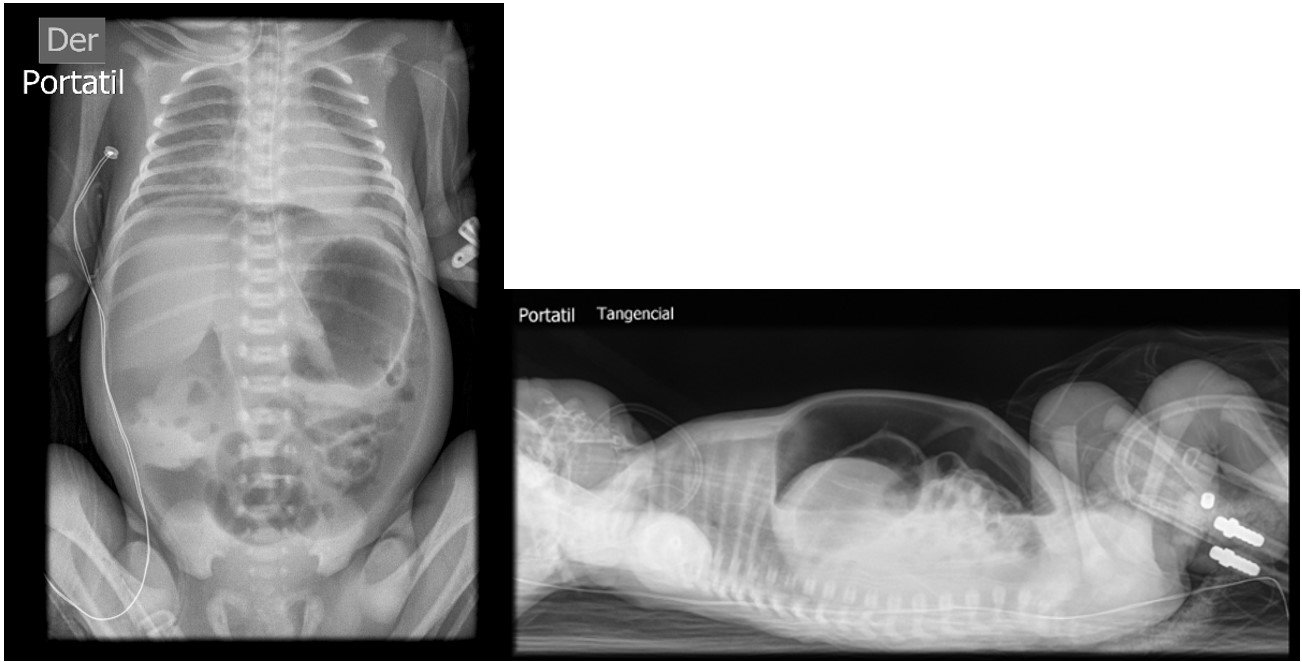
On the fourth postoperative day, the patient was extubated and had an appropriate respiratory pattern. She remained with a high-flow nasal cannula, continuing with the gastric rest and parenteral nutrition. Due to persistent abdominal distension despite the presence of an orogastric tube (OGT), an abdominal X-ray was requested, revealing marked pneumoperitoneum. She was reevaluated by a pediatric surgeon, who recommended exploratory laparotomy due to suspected gastric suture dehiscence.
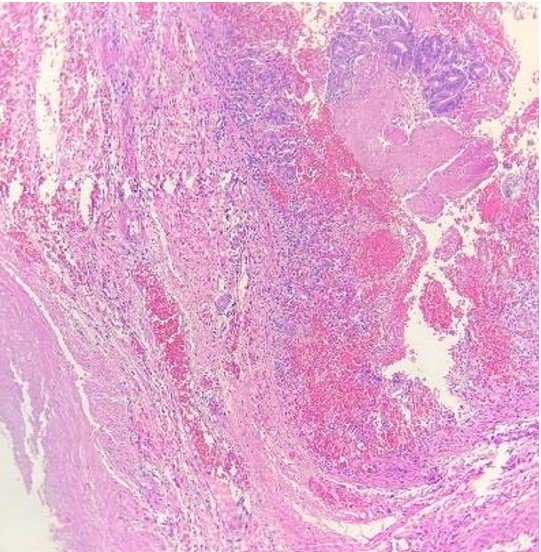
Due to alterations in her coagulation profile and a delay in obtaining informed consent, she was taken to the operating room at 11 hours after the diagnosis was confirmed. The previous gastric suture line was intact, but a new perforation of approximately 3 cm was identified at the gastric fundus, with free intestinal fluid in the cavity. The perforation was sutured in two layers, and the cavity was irrigated with saline solution. A biopsy of the perforation edge was performed, and a Penrose drain was placed. In the immediate postoperative period, she was reintubated and returned to the NICU. The coagulation profile was corrected, and she received packed red blood cells and fresh frozen plasma. Despite the OGT, she experienced gastric distension, requiring gentle intermittent aspiration with a syringe. On the fifth postoperative day, an abdominal X-ray showed evenly distributed air in the intestinal loops and a normal gastric chamber. She passed meconium spontaneously, the Penrose drain was removed, her weight was 1.440 grams, and she experienced a bilious vomit. On that day, she was extubated and tolerated the procedure, remaining on high-flow nasal cannula. Antibiotics were discontinued after negative blood culture results. In the following days, she had occasional episodes of bilious vomiting, but abdominal X-rays did not show any abnormalities. On the ninth postoperative day, as she had minimal residual gastric content via the OGT, enteral nutrition was initiated and gradually increased until achieving full oral feeding by day 33. Parenteral nutrition was gradually tapered and discontinued by day 26. She was discharged definitively on day 34 of life with a weight of 2.050 grams. She continued to have outpatient follow-up for the diagnoses of a 1mm ventricular septal defect and retinopathy of prematurity. The pathology report of the muscle wall biopsy showed fibromuscular connective tissue with high cylindrical glandular presence, moderate luminal hemorrhage, and mild chronic inflammatory infiltrate (figure 4).
DISCUSSION
Neonatal gastric perforation is a potentially life-threatening condition. However, in recent years, survival rates of up to 93% have been achieved, as reported by Sakaria
(2);
This is likely due to early surgical intervention, as 86% of their patients received surgical treatment within the first 12 hours of symptom onset. In the case series presented by
Byun et al., the time between symptom onset and surgical intervention was identified as a prognostic factor for survival (9).
The case presented in this report underwent early surgical management on both occasions, within 12 hours of diagnosis, which possibly contributed to the favorable outcome.
A congenital defect in the gastric musculature has been considered as the determining factor in neonatal gastric perforation (10,11),
However, it has also been postulated that these changes may be solely due to gastric distension (12).
The pathological examination of the gastric wall in our patient showed the presence of musculature, ruling out this possibility as the cause of the perforation.
Multiple factors can cause neonatal gastric perforation, including mechanical factors such as the insertion of nasogastric or orogastric tubes, esophageal intubation, mechanical
ventilation, or the presence of congenital malformations that increase intragastric pressure (such as pyloric stenosis/atresia, duodenal atresia, jejunoileal atresia, tracheoesophageal
fistula, diaphragmatic eventration/hernia, intestinal malrotation with midgut volvulus, congenital defects of the abdominal wall, etc.). The genesis of neonatal gastric perforation
has also been attributed to severe inflammatory-infectious processes such as sepsis or necrotizing enterocolitis; the administration of drugs (corticosteroids, NSAIDs); and other
rarer causes such as lactobezoars (4).
Among the mentioned factors, our patient had an orogastric tube and received mechanical ventilation with an endotracheal tube. However, there are other elements that may have contributed
to the perforation, such as prenatal corticosteroid administration (dexamethasone), hypoxia due to respiratory distress syndrome, as well as prematurity and low birth weight, which are
identified as risk factors.
There are few reports of recurrent neonatal gastric perforation. The most recently found case was that of a premature neonate (27 weeks) with very low birth weight (1110g), who presented a
single perforation in the posterior wall of the gastric body on the second day of life. It was repaired primarily, but on the fifth postoperative day, abdominal distension and
pneumoperitoneum were found on abdominal radiography. Surgical findings revealed multiple perforations at the greater curvature of the stomach, which were resolved through sleeve
gastrectomy, with subsequent favorable evolution (6).
In 2015, Park et al. (7) reported on a newborn girl at 25 weeks gestation weighing 900g, whose premature birth was due to premature rupture of membranes and
chorioamnionitis. Following her birth, she required endotracheal intubation and positive pressure ventilation, and was admitted to the intensive care unit where surfactant and mechanical
ventilation were administered. Due to persistent patent ductus arteriosus, intravenous ibuprofen was prescribed. On the seventh day of life, she exhibited general deterioration, abdominal
distension, and respiratory distress. An abdominal X-ray revealed intraperitoneal free air. Multiple gastric perforations were repaired surgically. In the postoperative period, disseminated
intravascular coagulation and acute renal failure occurred. Three days after the surgery, the patient experienced vomiting of intestinal contents and blood, and an abdominal X-ray showed free
air in the peritoneal cavity. A new surgical intervention was not possible because of severe hypotension and disseminated intravascular coagulation. A Jackson Pratt drain was inserted under
ultrasound guidance into a collection identified on the posterior aspect of the stomach, leading to resolution of the pneumoperitoneum and initiation of oral feeding. In this case, the
recurrence of gastric perforation was not confirmed surgically. It was assumed, based on ultrasound characteristics, that it was a new gastric perforation; however, it could also have
been dehiscence of the previous repair (7).
Among the cases presented in their series, Byun et al. (9) mentioned the case of a patient of 24 weeks weighing 730g, who had a primary repair of gastric perforation
on the fifth day of life. On the third postoperative day, an infantogram and sonogram revealed peritoneal free air, and a radiological study suggested gastric outlet obstruction. The patient
underwent a second surgery, where another gastric perforation and antral prepyloric membrane were found. Primary repair of the perforation and Heineke-Mikulicz pyloroplasty were performed,
resulting in a favorable outcome.
Much earlier, in 1988, Valenzuela and Urcelay published the case of a male newborn at 36 weeks of gestational age, weighing 2800g, with Apgar scores of 9 and 9 at 1 and 5 minutes, respectively.
He received oral feeding and required phototherapy due to hyperbilirubinemia. At two days of age, he experienced bilious vomiting and abdominal distension. An abdominal X-ray revealed
pneumoperitoneum. During surgery, a perforation was found on the anterior surface of the stomach, adjacent to the greater curvature, which was repaired by suturing. In the postoperative
period, he received parenteral nutrition and started oral feeding on the fifth day. Due to significant gastric residue, a radiological study revealed duodenal obstruction.
He underwent a second surgery on the eighth day after the initial operation, during which a gastric and ileal perforation, and intestinal malrotation were discovered. The perforations
were sutured, a gastrostomy was performed, and a peritoneal drain was placed. The postoperative period was uneventful, with oral feeding initiated on the seventh day, and he was
discharged at 37 days of age. Twenty days later, he was readmitted due to adhesive intestinal obstruction and underwent surgical intervention. However, he died 36 hours later due
to septic shock (8).
These last two reports involve a distal obstruction factor beyond the stomach (duodenal obstruction and prepyloric membrane), which triggered the gastric perforations and their recurrence.
In the presented case, no anatomical factor representing an obstruction was identified. The surgical findings described permeability throughout the entire intestine. Furthermore, the patient
spontaneously passed meconium after the first surgery, indicating preserved intestinal transit.
We believe that the description of this clinical case allows us to emphasize the possibility of neonatal gastric perforation recurrence. Therefore, vigilance should be maintained during
the postoperative evaluation of these patients, considering this contingency, especially if one of the most important signs persists, such as gastric distension, as reported by Reyna
Sepulveda et al. (7).
We believe that this publication will contribute useful information for future research and will enhance the understanding and management of this pathology.
Authorship contributions: The authors state that they are the creators of the entire article.
Financing: This research did not receive any specific grant from any funding agency in the public, commercial, or nonprofit
sectors.
Declaration of conflicts of interest:
None of the other authors reported a conflict of interest related to this study. The authors certify that there is no
conflict of interest with any financial organization regarding the material discussed in the manuscript.
Received:
April 11, 2023.
Approved:
May 10, 2023.
Correspondent author:
Velarde Paredes, Percy Edilberto
Address:
Av. Bolivar S/N Cdra. 8. Pueblo Libre , Lima, Perú.
Cellphone:
951 666 026
E-mail:
percy_vel@hotmail.com, percyvel7@gmail.com
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES