CLINICAL CASE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2023 - Universidad Ricardo Palma10.25176/RFMH.v23i4.6050
SOLITARY FIBROUS TUMOR OF THE BRAIN: A CASE REPORT
TUMOR FIBROSO SOLITARIO CEREBRAL: A PROPÓSITO DE UN CASO
Valeria Antuanne Ortiz Ramos
 1
1
Nora Nicole Itusaca Dueñas
 1
1
Lucia Victoria Ulloa Ordoñez
 1
1
Ricardo Romulo Paredes Pacual
 2,a
2,a
Jonathan Joel Torres Canqui
 2,b
2,b
Leslie Estefany Yovera Orellana
 2,c
2,c
José Manuel Vela Ruiz
 1,3,a,d
1,3,a,d
1 Instituto de Investigaciones en Ciencias Biomédicas (INICIB), Ricardo Palma University, Lima, Perú.
2 Clinical Pathology Unit of Emergency at Villa El Salvador Hospital.
3 Oncology Unit at San Juan de Lurigancho Hospital.
a Oncologist
b Radiologist
c Anatomic pathologist
d Master’s Degree
ABSTRACT
Introduction: Solitary fibrous tumors (SFTs) are rare mesenchymal neoplasms that, although typically develop in the visceral pleura, occasionally occur in the intracranial cavity. Furthermore, they are characterized by high rates of metastasis and recurrence.
Case Report: We present the case of a 59-year-old male patient with a 3-month history of headache and bradyphrenia. Computed tomography revealed a neoformative tumor infiltrating the nasal cavity, ethmoid sinuses, and anterior cranial fossa, involving the left frontal lobe. The patient underwent two exploratory craniectomies, during which diagnoses suggestive of high-grade glial neoplasia and SFT were made. For precise diagnosis, immunohistochemistry was performed, which was consistent with solitary fibrous tumor. The case is analyzed, focusing particularly on histopathological aspects, the unusual location of this tumor, and its variable clinical manifestations.
RESUMEN
Introducción:
Los tumores fibrosos solitarios (TFS) son neoplasias raras de origen mesenquimal que, aunque generalmente se desarrollan en la pleura visceral, ocasionalmente se presentan en la cavidad intracraneal. Además, se caracterizan por altas tasas de metástasis y recurrencia.
Caso clínico:
Se presenta el caso de un paciente masculino de 59 años con cuadro de 3 meses de cefalea y bradipsiquia. La tomografía reveló una tumoración neoformativa que infiltra cavidad nasal, celdillas etmoidales, y fosa craneal anterior, comprometiendo lóbulo frontal izquierdo. El paciente fue sometido a dos craniectomías exploratorias donde se realizaron los diagnósticos sugestivos de neoplasia glial de alto grado y TFS. Para la precisión diagnóstica se realizó inmunohistoquímica que fue compatible con tumor fibroso solitario. Se analiza el caso centrándose particularmente en los aspectos histopatológicos, localización inusual de este tumor y sus manifestaciones clínicas variables.
Solitary fibrous tumors (SFT) are rare neoplasms of the fusiform cells derived from dendritic mesenchymal cells. Although they mainly affect the visceral pleura, they have also been described in various locations including the intracranial cavity, which represents around 0.4% of all primary brain tumors. They are characterized by high rates of local extracranial metastasis, and persistent risk of recurrence even 10 years after the initial resection(1, 2).
The tumor often affects adults in their fourth to sixth decade of life. According to the age of presentation, SFT are divided into infantile (congenital) and adult type. The infantile appearance is extremely rare and until now less than 20 cases have been reported. The SFT show a low predilection for the masculine sex, which are associated with a greater risk of metastases and a shorter disease-free progression survival (3, 4).
In this case report we present a male patient of 59 years of age with a solitary fibrous intracranial tumor of difficult diagnosis and the importance of the anatomic pathological and immunohistochemical studies for its precision.
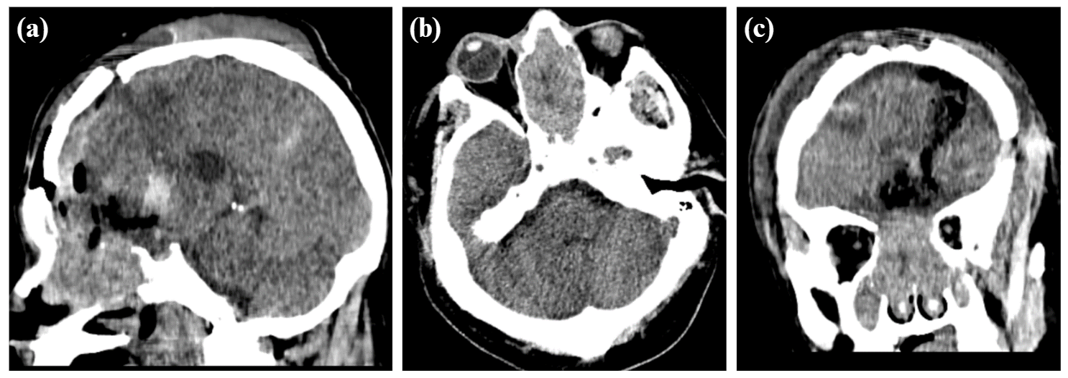
Male patient, 59 years old, with a history of high blood pressure under treatment and basal ganglion stroke in 2017 with sequelae (dysarthria, hemiparesis). He attends an outpatient visit in neurosurgery (01/04/2023) due to headaches of 3-month duration that intensified during the 2 weeks prior to admission, associated with bradyphrenia. In the brain computed tomography without contrast (12/20/22) a neoformative tumor was observed, which extended from the nasal cavity, ethmoid sinuses and anterior cranial fossa, invading the left frontal lobe with a midline shift (Figure 1), which is why a supra and infratentorial exploratory craniectomy was performed (01/23/23) where a tumor was observed of firm, septated consistency with abundant vascularization, adhered to the midline and orbital roof with continuity towards nasal cavities and paranasal sinuses.
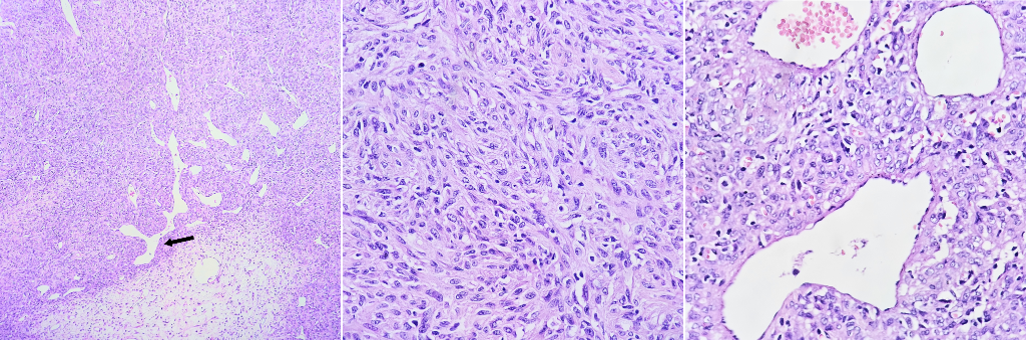
In the pathological anatomy (02/13/23), cerebral tissue was observed diffusely infiltrated by tumoral cells in a solid configuration with oval to elongated nuclei, moderate pleomorphism, cytoplasm with undifferentiated borders, presence of microvascular proliferation, mitotic index 12/10 cap. Findings were compatible with infiltrating malignant neoplasm, suggestive of high-grade glial neoplasm, furthermore an immunohistochemistry study is recommended for diagnostic accuracy.
A second craniectomy was performed (02/20/23) for the excision of the supratentorial residual tumor where a tumor lesion is observed in the anterior base and superior third of the nasal cavity with relation to a very vascularized fibrous frontonasal tumor, which is extracted. The pathologic anatomy (Figure 2) showed a tumor with a uniform hypercellularity, round to oval nuclei, with moderate pleomorphism, intratumoral ramified vessels (in staghorn appearance). Histological findings were compatible with a solitary fibrous tumor, immunohistochemistry is recommended.
In outpatient oncology consultation (04/03/23) a CT is observed with surgical sequelae at the left frontal lobe level with a residual tumoral mass of 3x3x1cm that infiltrates the cribriform plate and nasal cavity, local laminar hemorrhagic traces, the remainder without focal lesions, no hydrocephalus, with mild midline shift. Magnetic resonance (05/23) with contrast shows a residual tumor predominantly in the right nasal/ethmoid due to surgical treatment. On the other hand, the immunohistochemistry (08/07/23) showed results compatible with grade 1 solitary fibrous tumor according to the World Health Organization (WHO) classification, where a Ki67 was found with proliferative index <5%, CD34 and STAT-6 positive, EMA and GFAP negative. Furthermore, in histochemistry, an enhancement of pericellular deposits of reticuline fibers were found. The case was sent to the specialized institute for radiotherapy management of the residual tumor.
DISCUSSION
Solitary fibrous tumors (SFT) are rare mesenchymal neoplasms with fibroblastic differentiation, which represent a clinical challenge due to its local aggressive behavior, tissue infiltration, its recurrence tendency, and its potential to generate metastases in an indolent and late manner. The identification of new anatomical findings of these lesions complicates the differentiation between primary disease and metastases(5, 6).
SFT and hemangiopericytomas (HPC) were previously classified as separate tumors. However, after the finding of the fusion gene NAB2-STAT6 in 2013, SFT as well as HPC were treated as one entity according to the WHO classification for tumors of soft tissues. We can find diverse variants associated to different grades of aggressiveness, affected organ or metastasis (7, 9).
3 classification systems exist (Table 1) with the most updated one being Marseille Grading System (MGS) where grade 1 is defined by <5/10 HPF independently of necrosis; grade 2 has mitotic activity ≥5/10 HPF without necrosis and grade 3 has one mitotic activity of ≥5/10 HPF with necrosis. Furthermore, it was observed that the grade of the tumor, the mitotic count and the extension of resection were independent prognosis markers of progression-free survival(10).
These lesions can affect cranial meninges (including locations within brain tissue and the cranial base) as well as the spinal meninges (involving the nerve roots). Most tumors have signs and symptoms according to their location within the central nervous system and the effect of the mass due to its size. Furthermore, the more aggressive ones can be prone to hemorrhage as in other high-grade lesions (11, 12).
Macroscopically, they present as soft and lobulated tumors.
|
WHO 2016 |
MGS 2012 |
MGS 2019 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
GRADE I |
MGS I |
MGS I |
|||||||||
|
GRADE II |
MGS IIa |
MGS II |
|||||||||
|
GRADE III |
MGS III |
MGS III |
*10 HPF (MGS): counting of 10 adjacent fields with total magnification of 400x (total surface: 2.2 mm2) in the most proliferative areas as assessed in a H&E stained slide or guided by Ki67 immunohistochemical staining if available.
The 2016 WHO classification does not provide a definition for hypercellularity and “10 HPF”. (Modified by Macagno N. et al) (10)
While microscopy can distinguish between hypocellular variants, characterized by unorganized disposition of ovoid fusiform cells with dilated vessels and thin walls in a “deer horn” shape within the collagen matrix, and the densely cellular with round or ovoid cells with little collagen and less pronounced vessels. The prominent basal lamina that is illustrated with reticulin or IV collagen stain helps to differentiate them from meningiomas. In the immunohistochemical studies are frequently CD34 positive and epithelial membrane antigen (EMA) negative(13, 14).
The preoperative diagnosis of SFT depends mainly in the findings of the magnetic resonance (MR), since these tumors showed high vascularity and possible vessel leaks, which makes them hyperintense and easily detectable with gadolinium in T1 and T2-weighted imaging. However, the image characteristics seem to be similar to meningiomas which could lead to erroneous diagnosis. It was observed that the volume of the tumor, the tail sign and the analysis of the ADC map histogram may differentiate meningiomas from SFT. The capacity of the positron emission tomography (PET) to detect SFT is variable, and while it shows certain aspects, it should not replace MR (15, 17).
After diagnosis, the complete removal of the intracranial SFT is the gold standard treatment, followed by fractioned radiotherapy. It has been observed that preoperative arterial embolization may significantly reduce the risk of intraoperative massive hemorrhage and improve surgical safety. Adjuvant radiotherapy benefits patients with subtotal resection in terms of local control. In case of recurrence, stereotactic radiotherapy is safe and effective for grade 1 or 2 SFT, but not recommended for grade 3. Intensity-modulated radiotherapy is applied with doses according to grade or residual tumoral presence. Follow-up requires MR every 3-6 months in the first year. Aggressive SFT should have an annual extracranial tomography to detect extraneural metastases (16, 18).
With respect to gamma knife radiosurgery (GKRS) is still being studied but represents a reasonable tool to treat focal recurrence of small volume in patients with SFT, since it reduces size. GKRS is attractive because it limits the administration of doses to adjacent critical neurovascular structures (19).
Until now no chemotherapeutic agent has been approved due to lack of effectiveness. Traditional cytotoxic agents are used such as doxorubicin, ifosfamide, and taxanes, with limited effectiveness. The association of temozolomide plus bevacizumab is more effective and presents minor secondary effects. Other options include tyrosine kinase inhibitors such as sunitinib and sorafenib (20).
Metastases can occur even after total removal, on average at 7.5 years. After 10 years, with adequate follow-up, there is a 70% probability of recurrence or metastases, usually in bone, liver, or lung. The prognosis is generally bad, but several factors affected the effectiveness of surgical treatment, especially the size of the tumor and moment of diagnosis (20).
The prognosis of SFT has improved through a stratified risk model that predicts the probability of metastasis. This model uses four variables: age, tumor size, mitosis count, and presence of necrosis. With this information, it is possible to significantly differentiate the different risk groups, which allows a more precise evaluation of the course of disease (21).
The presentation of this case of solitary fibrous tumor in the intracranial cavity highlights the complex identification of this uncommon neoplasia. Its unusual location, variable clinical manifestations and its high tendency of recurrence and metastasis makes its management a clinical challenge.
Authorship contributions:
All the authors participated in the conceptualization, investigation, methodology, resources and drafting of the original manuscript.
Financing:
Self-financing.
Declaration of conflict of interest:
The authors declare not having any conflicts of interests.
Recevied:
October 29, 2023
Approved:
November 11, 2023
Correspondence author:
Valeria Antuanne Ortiz Ramos.
Address:
33, Av. Alfredo Benavides 5440, Santiago de Surco 15039
Phone:
(+51) 944467529
E-mail:
antuanneortiz2@gmail.com
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES