CLINIC CASE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2024 - Universidad Ricardo Palma10.25176/RFMH.v24i2.6098
PULMONARY ASPERGILLOSIS, AN APPROACH TO CLINICAL AND RADIOLOGICAL MANIFESTATIONS. REPORT OF THREE CASES
MANIFESTACIONES CLÍNICAS Y RADIOLÓGICAS DE LA ASPERGILOSIS PULMONAR: INFORME DE TRES CASOS
Onaldo José Barrios Taborda
 1,a
1,a
Jhon Edward Valencia Marulanda
 2,b
2,b
Luisa Fernanda Sierra Garzón
 2,b
2,b
Andrés Alirio Restrepo Bastidas
 2,b
2,b
Mateo Aguirre Flórez
 2,b
2,b
Ángela María Giraldo Montoya
 3,4,c
3,4,c
1 Universidad Simón Bolívar. Barranquilla, Colombia.
2 Grupo Investigación de Medicina Crítica y Cuidados Intensivos GIMCCI, Universidad Tecnológica
de Pereira. Pereira, Colombia.
3 Universidad Tecnológica de Pereira. Pereira, Colombia.
4 Universidad de la Sabana. Pereira, Colombia.
a Estudiante de Postgrado
b Investigador
c Internista, Neumóloga
ABSTRACT
Pulmonary aspergillosis, caused by the opportunistic fungus Aspergillus, primarily affects
immunocompromised individuals. This report presents three cases: An 18-year-old female with acute
leukemia developed respiratory distress and bilateral "tree-in-bud" patterns on CT. Despite voriconazole
treatment, she succumbed to respiratory failure. A 58-year-old female with diabetes and COPD had dyspnea
and hemoptysis. Imaging revealed a cavitated lesion, confirming aspergilloma. Surgery was considered due
to active hemoptysis. A 41-year-old female with a history of tuberculosis presented with fever and
respiratory symptoms. CT showed cavitated lesions and bronchiectasis, confirming chronic aspergillosis.
She responded well to voriconazole. These cases highlight the variability in pulmonary aspergillosis and
underscore the importance of timely diagnosis and treatment to improve patient outcomes.
Keywords: Aspergillosis, Pulmonary; Aspergillus; Immunocompromised Host; Voriconazole;
Respiratory Tract Infections (source: MeSH NLM)
RESUMEN
La aspergilosis pulmonar, causada por el hongo oportunista Aspergillus, afecta principalmente a
individuos inmunocomprometidos. Este reporte presenta tres casos: Una mujer de 18 años con leucemia
aguda desarrolló dificultad respiratoria y patrones bilaterales de "árbol en brote" en la tomografía
computarizada (TC). A pesar del tratamiento con voriconazol, falleció debido a insuficiencia
respiratoria. Una mujer de 58 años con diabetes y EPOC presentó disnea y hemoptisis. Las imágenes
revelaron una lesión cavitada, confirmando un aspergiloma. Se consideró la cirugía debido a la
hemoptisis activa. Una mujer de 41 años con antecedentes de tuberculosis presentó fiebre y síntomas
respiratorios. La TC mostró lesiones cavitadas y bronquiectasias, confirmando aspergilosis crónica.
Respondió bien al voriconazol. Estos casos destacan la variabilidad en la aspergilosis pulmonar y
subrayan la importancia de un diagnóstico y tratamiento oportunos para mejorar los resultados en los
pacientes.
Palabras clave: Aspergilosis pulmonar; aspergillus; huésped inmunocomprometido; voriconazol;
infecciones respiratorias (fuente: DeCS-
BIREME)
INTRODUCTION
The biologist Pier Antonio Micheli first described the genus Aspergillus in 1729. Aspergillus is a
filamentous fungus with a universal distribution in the environment and is an example of an
"opportunistic pathogen". This article focuses on pulmonary aspergillosis, an infection caused by this
fungus in the respiratory tract (1). The populations most frequently
affected include immunosuppressed patients with hematological malignancies, transplant recipients,
patients undergoing immunosuppressive therapy, those with connective tissue diseases, Chronic
Obstructive Pulmonary Disease (COPD), Diabetes Mellitus type 2 (DM type 2), sustained neutropenia for
more than ten days, innate and acquired immunodeficiencies, and those on chronic steroid use for more
than three weeks (2, 3). Invasive Aspergillus infections
have reported mortality rates of up to 50-85% in their invasive form (4).
This study will analyze various syndromes of pulmonary aspergillosis, which present with different
clinical and radiological manifestations. Upon suspecting the diagnosis, several approaches can be used,
including serum antigen detection and bronchoalveolar lavage (BAL) antigen detection (5). The U.S. Food and Drug Administration (FDA) considers an optical density
(OD) index of ≥0.5 to be positive for the galactomannan enzyme immunoassay (EIA) in both serum and BAL
fluid, although a revised threshold of 1.0 for BAL fluid is now included in the European Organization
for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium
(EORTC/MSGERC) definitions (6, 7).
CASE SERIES
The following are 3 cases of patients with pulmonary aspergillosis and their radiological features.
Informed written permission was obtained from each patient to publish this case report for academic
purposes. In the case of patient #1, who died, informed consent was provided by her parents.
Case #1
An 18-year-old female patient with no prior pathological history presented with weight loss of 8 kg over
one month and febrile peaks. Extension studies documented neutropenia and hepatosplenomegaly. Due to a
suspicion of hematologic pathology, a bone marrow aspirate showed 9.4% blasts and 61% eosinophils, while
a bone marrow biopsy revealed 31% blasts, compatible with acute lymphoblastic leukemia. Targeted
chemotherapy for her oncologic disease was initiated. During follow-up, she experienced febrile peaks,
dyspnea, and tachypnea, with chest X-ray findings of pulmonary opacities and persistent symptoms despite
broad-spectrum antibiotic treatment with Meropenem (a carbapenem) and negative blood and urine cultures.
High-resolution CT (HRCT) of the chest was requested, revealing images of a bilateral "tree-in-bud"
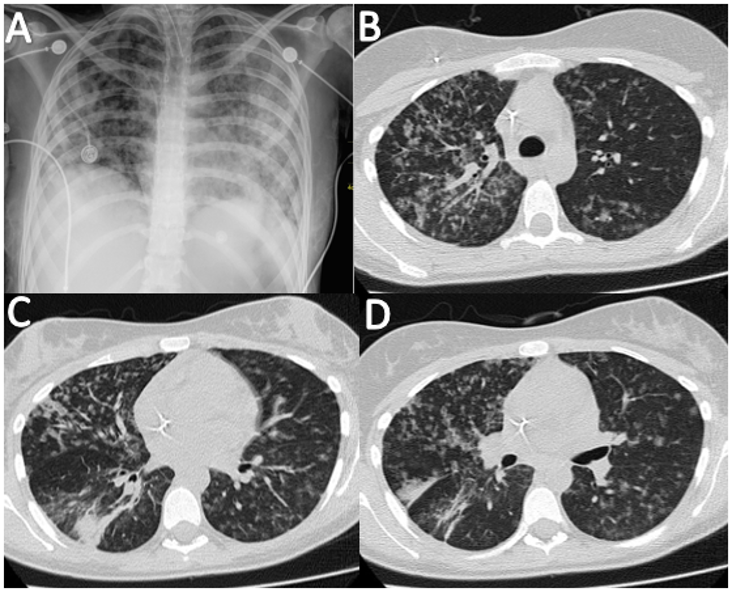
pattern, leading to a suspicion of angioinvasive pulmonary aspergillosis (Figure 1).
A. Chest X-ray showing reticulonodular opacities with bilateral distribution.
B-D. Computed axial tomography of the thorax showing a tree-in-bud pattern.
Figure 1. Imaging Studies of an 18-year-old Female with Acute Lymphoblastic Leukemia and Suspected Pulmonary Aspergillosis
Fibrobronchoscopy was performed, and a positive endobronchial sample for galactomannan (2.0) was
obtained. Therapy with voriconazole was started with a loading dose of 6 mg/kg every 12 hours on the
first day, followed by 4 mg/kg every 12 hours. The patient showed partial recovery in the first few
days. However, given the extent of the disease and her underlying hematologic neoplasia, she had an
unfavorable clinical course. She was admitted to the intensive care unit (ICU) for worsening respiratory
failure, required invasive mechanical ventilation, and subsequently expired.
Case #2
A 58-year-old female patient with a history of type 2 diabetes and GOLD B COPD reported respiratory
symptoms for two years, including dyspnea and occasional expectoration of "pints of blood" sputum. She
consulted for increased dyspnea, worsening hemoptysis, fever, chills, and malaise. Inhalation therapy,
oxygen, and systemic corticosteroids were initiated. Suspecting superinfection, antibiotic therapy with
Ampicillin/Sulbactam and Clarithromycin was started, resulting in partial improvement. Chest X-ray
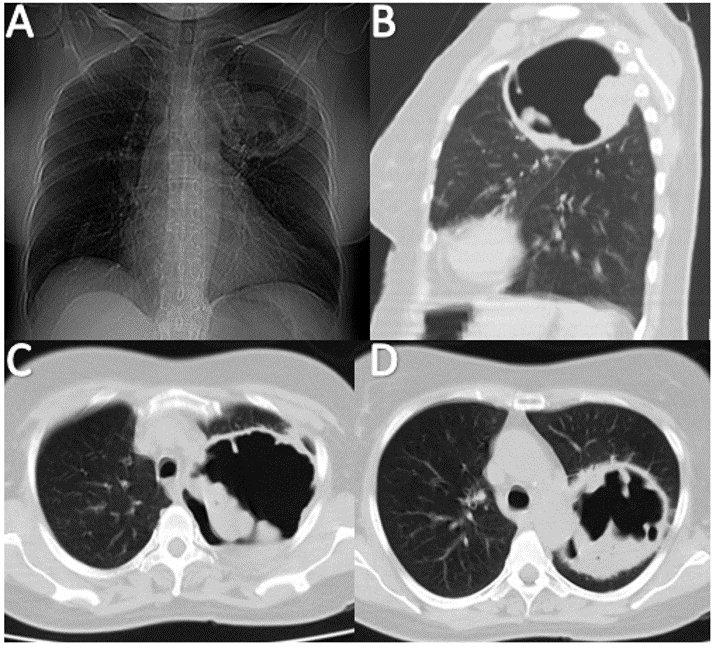
revealed increased lung volumes (air trapping) and a cavitated image in the left upper lobe (Figure 2),
prompting the initiation of broad-spectrum antibiotic treatment. HRCT showed signs of centrilobular
emphysema and a large cavitary image with dense internal material, suggesting an aspergilloma
(mycetoma).
A. Chest X-ray showing a cavitated lesion in the left upper lobe with thick, scalloped borders and internal radiopacity.
B-D. Computed axial tomography of the thorax showing a cavitated lesion with thick borders, irregular interior borders, and high attenuation material inside.
Figure 2. Imaging Studies of a 58-year-old Female with COPD and Suspected Aspergilloma
Fibrobronchoscopy with bronchoalveolar lavage was performed, yielding a positive Aspergillus
Galactomannan Antigen result (1.9). Given the positive radiological and serological findings, chronic
pulmonary aspergillosis (simple aspergilloma) was diagnosed. A thoracic surgery evaluation was requested
to consider a left upper lobe lobectomy due to the lesion's size and active hemoptysis. Antifungal
treatment was not initiated due to poor antifungal tissue penetration.
Case #3
A 41-year-old female patient from Ecuador, residing in Colombia for 19 years, with a history of
pulmonary tuberculosis treated for six months in 2014, presented with one week of unquantified fever,
hyporexia, headache, wet cough with abundant whitish expectoration, asthenia, adynamia, extreme
weakness, and dyspnea. Upon admission, she was considered to have an acute community-acquired infectious
process and was started on broad-spectrum Beta-lactam (Ampicillin-Sulbactam 1.5 g IV every six hours)
and inhalation therapy. Chest X-ray showed interstitial opacities, bilateral apical fibrous tracts, and
apical cavity-type findings. Due to poor clinical evolution, a contrasted chest tomography and molecular
tests (GeneXpert in sputum: negative for BAAR) were requested.
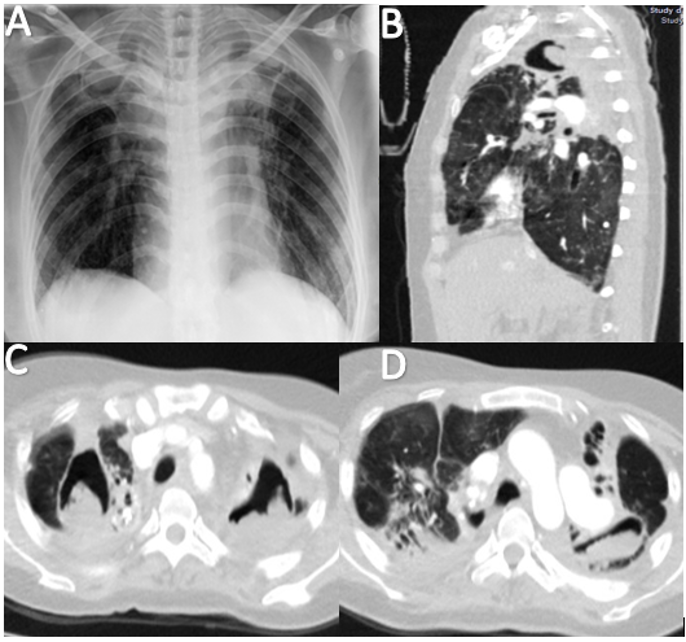
A. Chest X-ray showing thickening of apical caps, superior hilar traction, and upper lobe volume loss.
B-D. Computed axial tomography of the thorax showing cavitated lesions with high attenuation material inside, bilateral apical traction bronchiectasis, and signs of honeycombing in the lingular segments.
Figure 3. Imaging Studies of a 41-year-old Female with a History of Pulmonary Tuberculosis and Chronic Cavitary Pulmonary Aspergillosis.
The chest CT scan official result indicated hypodense, rounded images with well-defined regular contours
of thick-walled cavities surrounded by hyperdense areas. Bilateral apical traction bronchiectasis and
some areas of honeycombing were noted in the lingular segments, along with ground-glass opacities in the
remaining lung fields (Figure 3). Fibrobronchoscopy isolated multi-sensitive Pseudomonas aeruginosa,
with PCR for Mycobacterium negative and galactomannan in BAL positive (3.55), leading to a diagnosis of
chronic cavitary pulmonary aspergillosis. The patient was started on oral voriconazole 200 mg every 12
hours for six months. She evolved satisfactorily, was discharged, and completed treatment as an
outpatient.
DISCUSSION
Aspergillus is a ubiquitous and resilient organism, thriving best in humid environments, although spore
aerosolization and dispersal are most effective in dry climates. Among the hundreds of Aspergillus
species, Aspergillus fumigatus is the most common pathogenic species in humans. The small size and
hydrophobicity of its spores confer a dispersal advantage. Though less common, Aspergillus flavus and
Aspergillus niger also contribute to the burden of pulmonary aspergillosis. Inhaled spores settle by
sedimentation in the distal airways and alveolar spaces. In healthy individuals, spores are eliminated
by mucociliary clearance and immune defenses (8, 9).
The clinical presentation of invasive aspergillosis includes fever, cough, dyspnea, chest discomfort,
and hemoptysis. Chest CT is more sensitive than chest radiography. CT signs that constitute clinical
evidence of invasive lung disease, according to the 2008 criteria, include dense, well-circumscribed
lesions with or without a surrounding "halo" of ground-glass attenuation, a rising air sign, and cavity
formation. A retrospective study of chest CT images in 235 patients with invasive aspergillosis
demonstrated the presence of one or more macronodules (94%), halo (61%), consolidation (30%),
infarct-like nodules (27%), cavitary lesions (27%), and signs of rising air (10%) (10).
This clinical entity warrants consideration by pulmonologists and intensivists, as delayed diagnosis and
management can increase morbidity and mortality in patients with risk factors. From clinical suspicion
to the interpretation of images and treatment, managing this condition can be challenging. Therefore, a
comprehensive approach is essential.
Aspergillus is a common environmental organism. While pre-existing pulmonary disease or immune
dysfunction have been recognized as prerequisites for developing pulmonary disease in response to
Aspergillus, recent studies indicate that even a modest degree of immunosuppression increases this risk.
The type of pulmonary response often depends on host factors. Invasive pulmonary aspergillosis is
frequently found in patients with chronic lung disease exposed to oral or inhaled corticosteroids and
critically ill patients. Diagnosing invasive aspergillosis involves understanding the populations and
environments that predispose individuals to infection. Recognizing that positive cultures may indicate
invasive disease, noninvasive galactomannan tests can be helpful, though their sensitivity varies among
studies and their clinical utility remains unclear. Chronic cavitary pulmonary aspergillosis primarily
occurs in patients with pre-existing lung disease. Outcomes are generally poor without antifungal
treatment. Future research to identify the immune alterations that mediate inflammatory responses to
Aspergillus will enhance our understanding of the pathogenesis of these syndromes.
CONCLUSION
This case report highlights the variability in clinical and radiological manifestations of pulmonary
aspergillosis across three patients with underlying conditions, underscoring the complexity of its
diagnostic and therapeutic management. Although infrequent, this type of infection is critical for
diagnosis and management. Recent information suggests that this entity is underdiagnosed.
Authorship contributions:
OJB participó en la conceptualización, curación de datos, investigación, redacción -
borrador original y visualización. JEV participó en la conceptualización, curación de datos,
investigación, redacción - borrador original y visualización. LFS participó en la
conceptualización, curación de datos, investigación y análisis formal. AAR participó en la
conceptualización, curación de datos, investigación y análisis formal. MAF participó en la
revisión y edición del manuscrito y la traducción al inglés. ÁMG participó en la supervisión
y revisión y edición del manuscrito. Todos los autores aprobaron la versión final a
publicar.
Financing:
Self-funded
Declaration of conflict of interest:
The authors declare no conflicts of interest.
Recevied:
December 4, 2023
Approved:
January 15, 2024
Correspondence author:
Mateo Aguirre Flórez.
Address:
Cra. 27 #10-02, Pereira, Risaralda, Colombia.
Phone:
+57 6 1234567
E-mail:
maguirref96@utp.edu.co
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES