CLINICAL CASE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2023 - Universidad Ricardo Palma10.25176/RFMH.v23i4.6132
SARCINA VENTRICULI IN THE STOMACH:
AN UNEXPECTED AND POTENTIALLY DANGEROUS VISITOR
SARCINA VENTRICULI EN ESTÓMAGO: UN VISITANTE INESPERADO Y POTENCIALMENTE PELIGROSO
Eugenio Américo Palomino Portilla
 1,2a
1,2a
Hellen Johanna Gordillo de la Fuente
 3a
3a
Gabriela Jazmín Palomino Quiñones
 4b
4b
María del Pilar Quiñones Ávila
 2a,3a
2a,3a
1 Instituto de Investigación en Ciencias Biomédicas. Ricardo Palma University Lima, Perú.
2 National Hospital Edgardo Rebagliati Martins.
3 Faculty of Medicine Diagnosis SAC.
4 Faculty of Medicine, Ricardo Palma University. Lima, Perú.
a Pathologist
b Medical Student
ABSTRACT
Introduction: Sarcina ventriculi is a Gram (+), anaerobic, non-motile cocci, with a fermentative carbohydrate metabolism, that survives and grows without problems in environments with acidic pH. It is a known etiological agent in veterinary pathology, however its pathogenic role in humans is controversial. In recent years, the finding of this microorganism in different anatomical places has been reported in humans with increasing frequency, predominantly in the upper digestive tract, mainly in the stomach, in patients with dyspepsia and/or delayed gastric emptying, some of these cases with serious evolution, even fatal.
Clinical case: we report the case of a patient with dyspeptic symptoms, whose gastric biopsy identified Sarcina ventriculi and whose targeted pharmacological treatment ended the discomfort described. To the best of our knowledge, this is the first case reported in Peru.
RESUMEN
Introducción: Sarcina ventriculi es un coco Gram (+), anaerobio, inmóvil, con un metabolismo fermentativo de carbohidratos, que sobrevive y crece sin problemas en ambientes con pH ácido. Es un agente etiológico conocido en patología veterinaria, sin embargo, su rol patogénico en humanos es controversial. En años recientes, se ha reportado en humanos, cada vez con mayor frecuencia el hallazgo de éste microorganismo en diferentes lugares anatómicos, a predominio del tubo digestivo superior, principalmente en el estómago, en pacientes con dispepsia y/o retardo en vaciamiento gástrico, algunos de éstos casos con evolución grave, incluso mortal.
Caso clínico: Reportamos el caso de una paciente con sintomatología dispéptica, en cuya biopsia gástrica se identifica Sarcina ventriculi y cuyo tratamiento farmacológico dirigido terminó con las molestias descritas. A lo mejor de nuestro conocimiento, éste es el primer caso reportado en Perú.
Sarcina ventriculi (SV), also known as Clostridium ventriculi, is a Gram-positive anaerobic coccus, immobile, with a carbohydrate fermentative metabolism, surviving and growing in acidic environments (1). SV was first described in 1842 by Scottish microbiologist and anatomist John Goodsir, who microscopically analyzed the emetic content of a 19-year-old patient, subsequently described as sarcinae vomit (2). SV belongs to the Clostridiaceae family and takes its name from the Latin word "sarcina," meaning "package," as this bacterium typically arranges itself in tetrads or octets (3)
The tetrad arrangement occurs because the organism replicates in two planes (4), which can occasionally resemble plant material due to the refractive nature of its cell wall(5). This cell wall is a thick, fibrous layer, measuring 150 to 200 nm in thickness, predominantly composed of cellulose, a feature not described in other species and characteristic of SV (6).
This microorganism is commonly found in soil and air, where it can survive in spore form at alkaline pH (7). Originating from the soil, it is likely ingested with particles present in food1. The growth of SV in the human stomach is associated with delayed gastric emptying in conditions such as diabetic gastroparesis, gastric surgery, pyloric stenosis, or an obstructive mass. It has been identified in the stomachs of patients with gastric and pancreatic adenocarcinoma (4). In these diseases, the acidic gastric pH, the presence of carbohydrates, and other nutrients in food, allow SV to rapidly multiply1. Lam-Himlin et al. suggest that a pre-existing defect in the mucosa provides a niche for the development of emphysematous gastritis, rather than a direct invasion of SV into the gastric wall (4).
SV is commonly considered a veterinary pathogen, affecting livestock, cats, and horses8, where it often causes gastric dilation and death (9). The pathogenic role of SV in humans is unclear and debated, having been isolated from the feces of healthy individuals predominantly consuming a vegetarian diet (10).
The focal accumulation of acetaldehyde and ethanol formed by the fermentation of carbohydrates could induce gastro-duodenal injury similar to the damage caused by acute alcohol intake. Furthermore, the production of carbon dioxide by glucose fermentation and pyruvate metabolism causes abdominal distension in some patients (11).
Recently, various reports have shown an association between SV in the stomach with dyspepsia, nausea, abdominal pain, and gastric ulcer4, rarely emphysematous gastritis (12), and gastric perforation (11). However, SV has also been found in stomachs without any pathological changes4. Pediatric cases are less common, but they have a higher risk of complications such as emphysematous gastritis or gastric perforation (12).
Endoscopy usually reveals food retention, stenosis, necrosis, or mucosal ulceration (13), but there are no specific data for the diagnosis of SV infection.
The histological features of the gastric mucosa can range from unremarkable to acute hemorrhagic gastritis and ulceration (11). The microorganisms are generally located near the mucosal surface, in the gastric mucus, rather than in the foveola and are not invasive (14). SV is recognized by hematoxylin-eosin for its morphological features: intense basophilia, refractory nature resembling plant material4, cuboidal shape, arrangement in tetrads1, individual size between 1-8 to 3 um (4), and flattening of its lateral walls in areas of contact with adjacent cocci11. It must be differentiated from Micrococcus spp, Gram-positive cocci that also grow in tetrads but measure 0.5 um in diameter (15), and from Sarcina maxima, which lacks the thick cellulose layer characteristic of SV (6).
The reports indicate successful eradication of SV using treatment with metronidazole or in combination with another antibiotic (11).
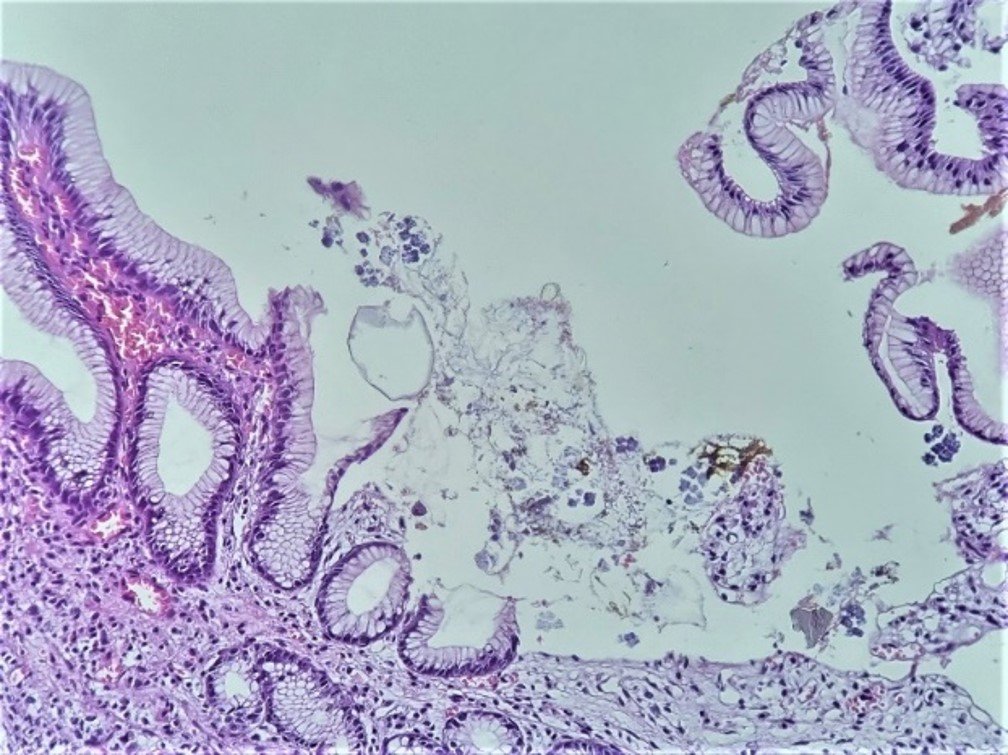
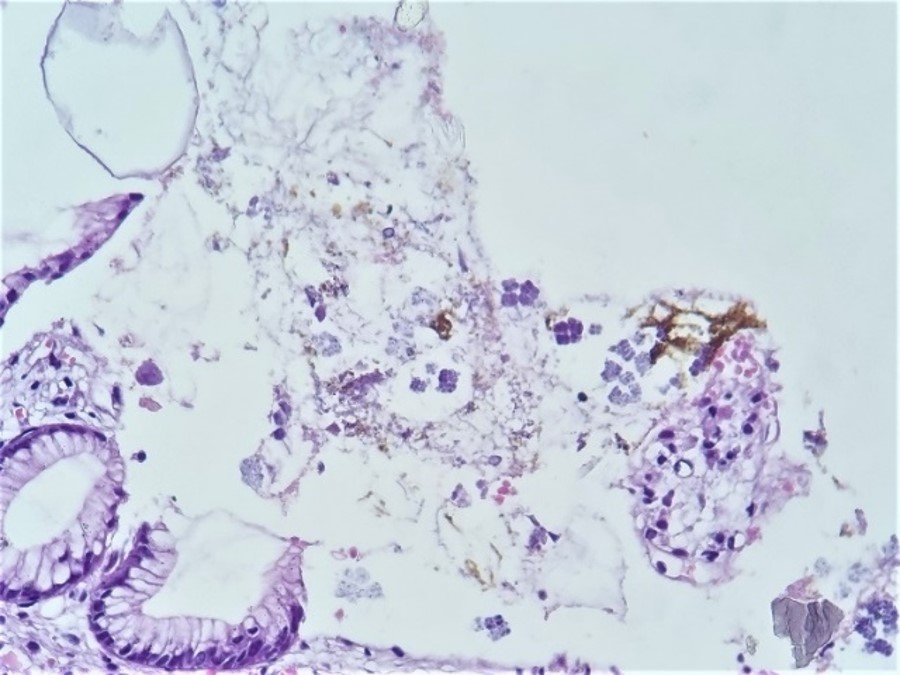
A 68-year-old female patient from Sullana, Piura, with no significant pathological history, presents with burning epigastric pain for the past two weeks, associated with hyporexia, food reflux, and diarrhea, without further details mentioned. She consults a general physician who, after a clinical examination, prescribes pharmacological treatment for Helicobacter pylori infection. After completing the treatment, the clinical picture persists. She then decides to consult a gastroenterologist, who performs an upper endoscopy and finds signs suggestive of gastritis, and proceeds with a biopsy. In the macroscopic study, 03 tissue fragments, measuring 3x3x2mm, whitish, elastic, are received. The histopathological examination identifies lymphocytes in the lamina propria and erosive areas. Dispersed in various places on the surface of the biopsies, quadrangular, basophilic, three-dimensional structures are identified, consisting of groups of 4 quadrangular formations, adjoined to each other, each individually covered by a noticeable refringent layer (Figure 1). In areas, these groups are arranged in octets, giving the approximate three-dimensional image of a cube, formed by 8 similar structures, in two planes. These structures are attached to the epithelial surface (intact or eroded) or free, occasionally within amorphous material related to partially digested food content (Figure 2). The approximate size of each individual structure is between 2 to 3 microns, and the cubic formation resulting from their union measures about 4 to 6 microns per plane. Noteworthy is the individual covering of each structure, constituting a kind of strongly refringent capsule. In areas, the tetrads and octets group together, resulting in complex assemblies easily visible under the microscope (Figure 3).
DISCUSSION
Sarcina ventriculi is a microorganism well-identified by its histological and microbiological characteristics, with a well-defined pathogenic role in livestock, cats, and horses, where it often affects the digestive tract, potentially causing distension, perforation, and death.
Its role as a human pathogen is controversial. However, in recent years, various authors have described the finding of SV in humans, primarily in gastric pathology with associated symptomatology, with some of these cases leading to serious complications. Until 2013, there were reports of only 8 cases, which increased to 65 cases by the year 2022.
To the best of our search and knowledge, this would be the first reported case in Peru, adding to a few other reported cases in neighboring countries.
The case involved a 68-year-old woman from northern Peru, who presented with dyspeptic symptoms and initially received anti-Helicobacter therapy without clinical improvement. Subsequently, a specialist conducted an endoscopy, took biopsies, and with the results, prescribed a successful targeted treatment. It is important to note that Sarcina ventriculi is an anaerobic coccus, which is why the reported therapeutic regimens are based on metronidazole. It was evident in this case that the initial therapy was ineffective, as anti-Helicobacter therapy does not affect anaerobic germs.
In the histopathological study, a mild to moderate chronic gastritis was found, with areas of discreet erosion, and in various superficial zones, the presence of SV exhibiting its striking morphological characteristics already described. The first observation from the panoramic view is the presence, on the surface of the biopsies, of intensely basophilic geometric structures. At higher magnification, the three-dimensional aspect of the tetrads or octets is identified, formed by tightly adjoined microorganisms, with flattening of their contours at contact points and separated from each other by a characteristic thick refractive layer. The finding is relatively simple, as smaller structures (such as Helicobacter pylori) are routinely identified. A relevant detail is that for the identification of SV, the usual hematoxylin-eosin staining is sufficient.
As previously described, conditions that favor the presence and proliferation of SV are delayed gastric emptying, associated with various causes, such as diabetic gastroparesis, previous surgery, stenosis for any reason, and mass effect by adjacent neoplasms. Considering the incidence in the Peruvian population of diabetes mellitus and neoplasms alone, an underdiagnosis of SV and its associated risks can be inferred. Additionally, given that the breeding of domestic cats is common in our population, their role as a potential reservoir and cause of contagion to humans should be evaluated.La utilidad del presente reporte es que permite conocer mejor a un microorganismo que cada vez se reporta más frecuentemente como causa de patología digestiva humana. Dada la gran cantidad de biopsias obtenidas por endoscopías altas y la incidencia de condiciones que retrasan el vaciamiento gástrico en la población peruana, sería interesante hacer un seguimiento y búsqueda de este microorganismo en particular, al cual se le ha relacionado con severas complicaciones.
Sarcina ventriculi can and should be identified in digestive biopsies, given that its distinctive morphological characteristics allow it, and it does not require special staining. The findings should be correlated with clinical and endoscopic data, as its presence can be associated with severe complications arising from its unique microbiological characteristics, such as being a Gram-positive anaerobic coccus, thriving and reproducing well in acidic environments, and using a fermentative carbohydrate metabolism that generates substances harmful to the epithelium and produces gas.
It is expected that, in the future, cases of SV in humans will be described more frequently, prompting a better study of the implications that this known veterinary pathogen can have on our population.
Authorship contributions:
EAP, HJG, GJP, and MPQ participated in the conceptualization, research, and writing - original draft. EAP, HJG, and MPQ also participated in supervision, validation, writing - review and editing, and contributed resources. All authors participated in approving the final version.
Financing:
The authors state that they have not received any type of funding for the preparation of this article.
Declaration of conflict of interest:
The authors declare no conflict of interest.
Recevied:
November 1, 2023
Approved:
December 5, 2023
Correspondence author:
Eugenio Américo Palomino Portilla.
Address:
Instituto de Investigación en Ciencias Biomédicas, Universidad Ricardo Palma, Lima, Perú
National Hospital Edgardo Rebagliati Martins, Lima, Perú
Phone:
998483195
E-mail:
eugenio.palomino@urp.edu.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES