ORIGINAL ARTICLE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2024 - Universidad Ricardo Palma10.25176/RFMH.v24i1.6189
COLD CHAIN OF THE PERUVIAN NATIONAL IMMUNIZATION PROGRAM IN THE CONTEXT OF THE COVID-19 PANDEMIC
CADENA DE FRÍO DEL PROGRAMA NACIONAL DE INMUNIZACIONES PERUANO EN EL CONTEXTO DE LA PANDEMIA COVID-19
Alfonso Gutiérrez-Aguado
 1,a
1,a
Mariana Mendoza
 2,b
2,b
Tatiana Sarazú
 3,a
3,a
Paula Rodriguez-Ordoñez
 4,a
4,a
1 Universidad Continental. Lima, Perú
2 Universidad Norbert Wiener. Lima, Perú.
3 Sanofi. Lima, Perú
4 Sanofi. Bogotá, Colombia
a Surgeon
b Nurse
ABSTRACT
Objective: The aim of this study was to identify critical aspects of the Cold Chain in the
immunization process in Peru.
Methodology: A descriptive study was conducted, analyzing data from the Ministry of Economy and
Finance (MEF) and the Ministry of Health (MINSA) for the years 2020-2021, as well as administrative
records from the MINSA's Regional Health Strategies for immunizations in 2020. Technical aspects
established in the Health Technical Standard for Cold Chain Management, such as obsolescence,
allocation, functionality, and storage capacity were taken into account.
Results: In the year 2020, at the national level, 61.8% of the cold chain equipment showed
obsolescence, with some regions exceeding 75%, with Lima’s metropolitan region being the most affected
at 88%. Concerning equipment allocation, 9% of the first-level health facilities lacked refrigeration
equipment, with Loreto having the highest deficit (46%), followed by Huancavelica with a 21% gap. The
overall equipment functionality nationwide was 84%, meaning that 16% of health facilities experienced
technical failures, affecting vaccine’s storage capacity and posing risks to their safety and
immunogenicity. Significant gaps were identified when considering quarterly or monthly storage for
COVID-19 vaccines or other health emergencies.
Conclusions: This study highlights potential risks in the operability and storage capacity of the
national immunization program's vaccines in Peru during contingencies such as the COVID-19 pandemic or
other health emergencies.
Keywords: Cold chain; Immunization programs; Immunization; Vaccination coverage; vaccines.
(Source: MeSH NLM).
RESUMEN
Objetivo: Identificar aspectos críticos de la Cadena de Frío en el Perú
Metodología: Estudio descriptivo. Se analizaron datos del Ministerio de Economía y Finanzas (MEF)
y del Ministerio de Salud (MINSA) de los años 2020-2021, así como los registros administrativos de las
Estrategias Sanitarias Regionales de inmunizaciones del MINSA en 2020. Se consideraron aspectos técnicos
de la Norma Técnica de Manejo de cadena de frío, como es obsolescencia, dotación, funcionalidad y
capacidad de almacenamiento.
Resultados: En el año 2020, en términos de obsolescencia el 61.8% de los equipos de cadena de
frío a nivel nacional presentaban obsolescencia, siendo regiones claves como Lima Metropolitana (capital
del país) la más afectada con un 88%. En cuanto a la dotación de equipos, el 9% de los establecimientos
de salud del primer nivel carecen de equipos de refrigeración, siendo Loreto la región con mayor déficit
46%, seguida de Huancavelica con un 21% de brecha. En términos de funcionamiento, se registra que el 84%
de los equipos a nivel nacional funcionan, y el 16% reportan fallas técnicas, lo cual representa alto
riesgo en la seguridad y potencia inmunogénica de las vacunas a prever. En términos de capacidad, al
considerar el almacenamiento trimestral o mensual para las vacunas contra la COVID-19 u otras
emergencias sanitarias se identificaron brechas significativas.
Conclusiones: Existen riesgos en la operatividad, suministro y capacidad de almacenamiento de las
vacunas del esquema nacional de inmunizaciones de Perú incluso ante emergencias sanitarias.
Palabras clave: Cadena de frío; Programa de Inmunizaciones; Inmunización, Vacunas. (Fuente: DeCS
BIREME).
INTRODUCTION
Vaccination is a successful and cost-effective public health intervention that directly reduces medical
costs (1). In 1896, Peru's public health system took three significant steps
in vaccination: 1)
mandatory vaccination and revaccination were implemented nationwide, 2) the Instituto Vacunal de Lima
(now known as the Instituto Nacional de Salud) was established, and 3) the Ministry of Promotion (now
the Ministry of Health) commenced its activities (2).
In 1974, the Pan American Health Organization (PAHO) through Resolution CD25.R27 established the
Expanded Program on Immunization (EPI), considering vaccination and epidemiological surveillance of
preventable diseases as fundamental strategies in 1977 (3). In 1979, EPI was
implemented in Peru, and
massive campaigns against poliomyelitis (National Vaccination Campaigns) were carried out successfully,
leading to the eradication of wild poliovirus in Peru by 1991, as well as measles in 2000 and rubella in
2006 (4).
Structural changes occurred in the Peruvian government from the year 2000 onwards, including
decentralization and regionalization (5). In health, "vertical" programs
like EPI were phased out,
leading to the creation of the National Immunization Health Strategy (ESNI, by its Spanish acronym) in
2004, with an Advisory Committee and a Permanent Committee as advisors (6).
The General Vaccines Law No.
28010 was enacted in June 2003, making vaccination activities mandatory and securing dedicated funds for
vaccination activities. An international evaluation in 2014 (7) led to the
integration of EPI into the
Ministry of Health (MINSA) structure (8), enhancing its significance.
The development of the cold chain has faced a series of challenges and solutions, propelling the
immunization process globally. In 1976, Professor David Morley of the Institute of Child Health in
London proposed that WHO establish a team within EPI to address three critical issues: the absence of
systems to monitor the temperature of heat-sensitive vaccines, lack of appropriate equipment for storing
and transporting vaccines, and insufficient adequately trained personnel to handle vaccines (9).
Morley emphasized the importance of proper cold chain management, while PAHO-UNICEF warned that
transportation issues, staff or vaccine shortages, or cold chain interruptions could lead to decreased
confidence and reduced demand for immunization services (10). This
underscores the need for robust
infrastructure and logistics to manage an efficient national immunization program.
In Peru's history, the Cold Chain has undergone several inventories. The first was conducted in 1989,
the second between 1993 and 1994, and the third in 2004 with UNICEF support, coinciding with the
establishment of the National Immunization Strategy and Vaccines Law. The latter inventory revealed the
need to revitalize immunization components, prioritizing the incorporation of new vaccines and upgrading
the Cold Chain with cutting-edge technology to ensure vaccine preservation and quality (11).
According to Shibeshi et al (12), vaccine supply within the cold chain can
be affected by geographical
inaccessibility to services, vaccine shortages, and/or cold chain issues, representing missed
opportunities for vaccinating children in many countries. Pambudi et al (13) state that the success of a
vaccination program relies not only on vaccine effectiveness but also on cold chain supply management.
Functionality, according to Ogboghodo et al (14), is a significant
determinant of cold chain management
practice, and Hatchett et al (15) describe that vaccine refrigerator
operation is crucial for ensuring
safe storage and maintaining efficacy.
Internationally, it is recommended that all three levels of the cold chain have appropriate management
and planning considering efficient vaccine supply management. According to WHO, for the introduction of
the COVID-19 vaccine (16), the cold chain is one of the operational
components that must be ensured for
planning, organizing, and executing a national vaccination against SARS-CoV-2, ensuring fair, equitable,
and safe vaccine access.
Tao et al (17) suggest exploring methods to calculate cold chain capacity
needs using immunization
product databases, considering that cold chain capacity needs for immunization programs can be
accurately measured with the volume of immunization product doses, as established in the Technical
Health Standard (NTS, by its Spanish acronym) for Cold Chain Management in Immunizations (18), whose
methodology has been employed for this study.
In the context of the COVID-19 pandemic, Peru aimed to vaccinate 24 million people (19), for which MINSA
secured procurement agreements under the COVAX Facility or bilateral agreements to ensure vaccine access
for the population, including migrants (20). During this period, technical
and operational guidelines
were established to implement vaccination, which were modified based on vaccine availability and
scientific evidence. Currently, Peru has four different vaccines against SARS-CoV-2 (21), which have
been distributed and administered nationwide.
The objective of this study is to identify critical aspects of the cold chain, using a structured
information database tool, whose analysis shows the situational reality in vital aspects of its
management and performance. This data, in the context of the COVID-19 pandemic and similar future
situations, will be crucial for timely and appropriate decision-making in national and subnational
immunization management by the Ministry of Health.
Materials and Methods
Location and Execution Period:
This study included information on cold chain equipment obtained nationwide from all MINSA health
service providers (IPRESS) that provided vaccination services during the period from December 2020 to
December 2021.
Study Type and Research Design:
Descriptive study analyzing databases from the Ministry of Economy and Finance (Integrated
Administrative Management System Asset Module and Integrated Financial Management System) (23) of MINSA
IPRESS, vaccine dose and distribution records (SISMED) (24) for the years
2020 and 2021, and
administrative records from MINSA's Regional Health Strategies for immunizations in 2020.
Study Variables:
Inclusion Criteria:
- Data on cold chain equipment from MINSA IPRESS providing vaccination services in 2020 and 2021, registered in MEF's SIGA Asset Module.
- Data on cold chain equipment from all MINSA IPRESS Management Units – UGIPRESS (25) registered in MEF's SIGA Asset Module for the years 2020 and 2021.
Data Collection Instruments:
This study established four evaluation criteria, aligned with the current Peruvian cold chain technical
standard (18). These were described based on data recorded in Regional
Health Directorates or Management
Entities (DIRESAs and GERESAs, by their Spanish acronyms), Integrated Health Network Directorates
(DIRIS, by its Spanish acronym), and provided by the SIGA Asset Module of the Ministry of Economy and
Finance.
Review of national and international technical information allowed for assessing the operability and
functionality of the Cold Chain for immunization in Peru in the context of the COVID-19 Pandemic:
Criterion 1: Obsolescence. Based on installation or usage time of equipment, this criterion
establishes
the adequacy of equipment for use. According to the Peruvian technical standard, all equipment should be
less than 10 years old to ensure proper functioning; otherwise, its usage relevance must be evaluated or
it should be withdrawn from service.
Criterion 2: Supply. This criterion identifies the number of Health Establishments equipped with
cold
chain equipment, highlighting the need for 100% equipment coverage nationwide. Each region vaccinates
100% of its first-level IPRESS, hence any establishment lacking this equipment poses a risk to vaccine
preservation.
Criterion 3: Functionality. This assesses the current condition or operating level of cold chain
equipment, which determines the operational status of the cold chain across all three levels of
healthcare. Non-functioning or repair-needing equipment does not ensure storage capacity.
Criterion 4: Storage Capacity by Levels. This describes the actual storage capacity of an IPRESS
to
cover routine vaccination needs and additional activities like COVID-19 vaccination. The standard
specifies that all vaccines must be stored between +2°C and +8°C at the local level, so all IPRESS must
have equipment for 100% of routine vaccines and vaccines for supplementary activities (campaigns,
emergencies, sweeps, among others).
Data Processing and Analysis Plan:
For data analysis and evaluation, information from the SIGA Asset database on cold chain equipment was
used, collected and reported for the years 2020 and 2021, alongside a comparative analysis based on the
description and location of these equipment types, functionalities, and lifespan per cost center
(IPRESS, UGIPRESS, Warehouse).
Processing and Analysis:
Data on electric and solar refrigerators and freezers were reviewed based on information obtained from
the MEF's SIGA Asset Module, chosen for consistency and possessing more evaluation and identification
variables than unofficial information available in most IPRESS vaccination service.
The SIGA Asset Module data was extracted from the cubes in the Cognos Power Play application (26).
Subsequently, information pertaining to evaluation criteria was exported to Microsoft Office Excel, and
report tables were processed using pivot tables. The final processing involved consolidating evaluated
variables by region (DIRESAs, GERESAs, DIRIS), by cost center (IPRESS, UGIPRESS), by category level, and
considering only variables identifying cold chain equipment used in immunizations. The analysis was
conducted based on the criteria established in the technical standard for cold chain management in
immunizations (18).
Ethical Considerations:
This research was conducted using freely accessible secondary databases from official state portals,
presenting no individual patient data. Therefore, approval from an ethics committee was not required.
Results
Obsolescence:
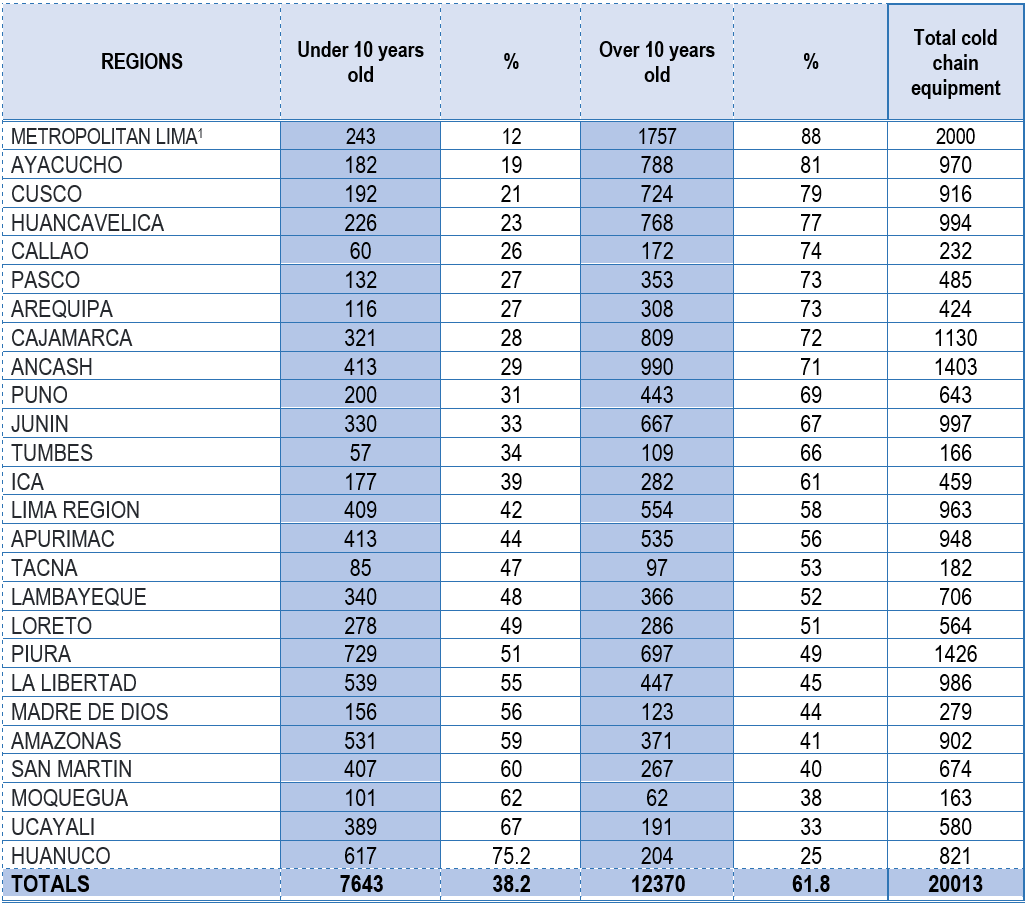
Using data from the SIGA Asset Module, the number and type of equipment older than 10 years were
identified. Table 1 reflects nationwide cold chain equipment that is obsolete; approximately 61.8% of
health facilities responsible for vaccination processes have obsolete cold chain equipment. In some
regions, this value exceeds 75%, notably Lima Metropolitan Area (88%), Ayacucho (81%), Cusco (79%), and
Huancavelica (77%), which are key regions in the country.
Source: SIGA Asset Module – MEF
The SIGA Asset data reports all active cold chain equipment in health facilities nationwide up to
December 2021, where 7,643 pieces of equipment (38.2%) are less than 10 years old, and 12,370 pieces of
equipment (61.8%) are over 10 years old. This finding is crucial in terms of risk management for cold
chain management in Peru.
Supply:
The supply of cold chain equipment in Peru has steadily increased since 2008. In 2021, during the
COVID-19 Pandemic, Peru strengthened its cold chain with an investment of 145 million soles (27),
increasing regional and local vaccine storage capacity. At a regional level, routine and COVID-19
vaccines can be stored globally; however, at the local level where vaccines are applied and must be
available, there are 727 first-level health facilities (9%) lacking this equipment as of December 2021,
representing an ongoing supply gap.
Table 2 shows that 91% of first-level health facilities have refrigeration equipment, while 727 (9%) do
not, with Loreto (46%) and Huancavelica (21%) regions having the largest gaps. These regions should be
prioritized for strengthening as they have dispersed and inaccessible populations, lower routine and
COVID-19 vaccination coverage rates, and report more outbreaks of vaccine-preventable diseases.
Source: SIGA Asset Module-MEF Data
Additionally, it is concerning that Lima, the capital, has one of the lowest supplies of cold chain
equipment despite being the most densely populated region. The lack of cold chain equipment leads to
inadequate capacity, potentially interrupting vaccination services and public health emergency responses
(30).
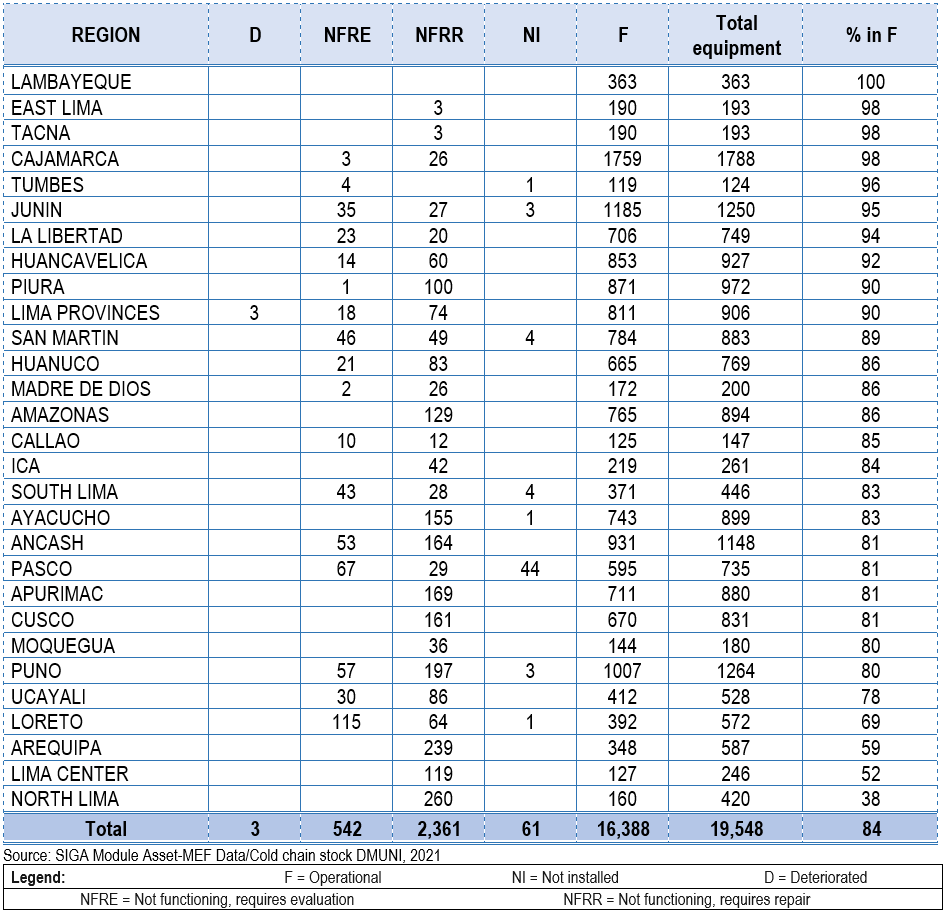
Functionality:
The functionality of national cold chain equipment (Table 3) is at 84%, meaning that despite 16% of
health facilities having refrigeration and freezing equipment, these have technical failures or
deterioration. As a result, the vaccine storage capacity is nonexistent in these health facilities,
posing a risk to the safety and immunogenic potency of the vaccines. In the context of COVID-19
vaccination or similar contingency situations, these establishments would struggle to store vaccines due
to the inability to guarantee proper temperature maintenance, which would affect the immunogenic
capacity of the vaccines. This situation jeopardizes the availability of routine vaccines (31) and
consequently leads to incomplete vaccination schedules or unvaccinated populations.
Table 3 shows that five regions have over 95% functionality of cold chain equipment (Lambayeque, East
Lima, Tacna, Cajamarca, and Tumbes), while five have less than 80% functionality (Ucayali, Loreto,
Arequipa, Lima Center, and North Lima), posing challenges in vaccine storage and vaccination operations.
Storage Capacity:
The National Technical Standard (NTS) for cold chain management in immunizations stipulates the need for
properly trained and qualified personnel to measure the volume of each vaccine in the National
Vaccination Scheme according to the methodology established in said standard (18). Table 4 shows that
while there is overall coverage for the storage capacity needed for regular schedule vaccines on a
quarterly and monthly distribution basis, when contrasted with the requirements for COVID-19 vaccine
storage, it is observed that 15 (58%) out of 26 regions can cover the quarterly and monthly storage
capacity needs for these vaccines. This analysis was conducted using information available as of
December 2021, without considering vaccines that require ultra-freezing storage, as these are only
stored at the national and regional levels (21). Additionally, the gap of
582,579 and 56,809 cubic
meters of quarterly and monthly storage capacity respectively for COVID-19 vaccines implies increased
rotation in the distribution of routine vaccines as well as COVID-19 vaccines in the 11 regions (42%)
that lack capacity for quarterly and monthly storage.
Table 4. Estimation of annual, quarterly, and monthly storage capacity needs for routine vaccines and gaps for COVID-19 vaccine storage based on available capacity with routine vaccines, December 2021
Source: SIGA Asset Module-MEF Data. Source MINSA: Estimation of COVID-19 vaccine needs - DMUNI / CENARES
Discussion
Currently, validation of timely specialized preventive maintenance control at the regional level of the
cold chain has not been achieved, resulting in equipment over 10 years old becoming planned
obsolescence, causing difficulties in storage capacity not only for regular schedule vaccines but also
for COVID-19 vaccines, as only 38.2% of equipment is less than 10 years old. Additionally, it is
noteworthy that around 80% of equipment in the top 5 regions of Peru is obsolete.
Cold chain interruption leads to avoidable vaccine wastage, and the COVID-19 pandemic demonstrated the
need for vaccines to be stored at different temperatures (between 0°C and 10°C, at -20°C and -70°C),
increasing the complexity of vaccine supply chains and emphasizing their importance. The current study
highlights the urgency and importance of promoting intervention strategies in refrigeration and
biological systems to enhance storage capacity, supply, and adequate vaccine management during future
contingencies similar to the COVID-19 pandemic.
Regarding supply, the study by Shibeshi, Masresha, and Daniel (12)
demonstrated that cold chain issues
accounted for the majority of missed vaccination opportunities for children in many countries, with 25%
of countries lacking sufficient cold chain equipment to execute extension services. Similarly,
Piché-Renaud (32), concerning vaccine supply, noted substantial
modifications to pediatric immunization
services in Canada due to COVID-19, necessitating strategies to mitigate immunization barriers during
the pandemic to prevent immunity gaps that could lead to an eventual increase in vaccine-preventable
diseases. Both situations described align with findings in Peru, where lack of equipment (9%) at the
primary care level jeopardizes supply of routine vaccines and COVID-19 vaccines to the population in
these areas. It also highlights that regions like Loreto, which have a high rate of morbidity and
mortality due to preventable infectious diseases, have a very low percentage of refrigeration equipment
supply (54%), exposing the population to higher risk.
The present study reveals a 16% malfunction rate in equipment, proving to be a critical factor in Peru's
cold chain management. According to Ogboghodo et al. (14), the presence of
functional refrigerators
(p=0.016) was the most significant determinant for cold chain management practices, and Hatchett
(15)
demonstrated that proper functioning of vaccine refrigerators and required health professional controls
ensures safe storage, maintaining efficacy. Therefore, as Feyisa (33)
identified, concerted efforts are
needed to provide proper vaccine cold chain management at immunization delivery points. Functionality
directly correlates with obsolescence, considering that a higher number of obsolete equipment at the
national level leads to greater dysfunctionality of these systems. Considering this criterion, the
results of this analysis are valuable, as the level of obsolescence of cold chain equipment in health
facilities responsible for vaccination exceeds 61%.
Regarding the storage capacity gap for COVID-19 vaccines, urgent measures are needed to avoid additional
costs due to high vaccine turnover that risks their safety and timeliness, as described by Ortiz et al.
(34) and Bulula et al. (35), who mention that
immediate attention to strengthening immunization systems
is essential to support pandemic responses, particularly enhancing vaccine storage capacity, which could
even reduce costs in vaccine supply chains. This study demonstrates the current collapse in monthly and
quarterly vaccine storage in Lima and Ucayali.
Conclusions
The analysis allows us to identify risks in storage capacity to safeguard vaccines in the national
scheme and to respond adequately to contingencies like the COVID-19 pandemic, which require timely
intervention. Considering that not all strategies and practices are suitable for every country and
circumstance (36), it is proposed to integrate approaches that
comprehensively address the current cold
chain issue for vaccines.
The cold chain is crucial in the vaccine supply system; the introduction of new vaccines challenges
storage capacity in the cold chain, necessitating innovations tailored to community needs, particularly
those of underserved populations (37). Patients expect to be treated with
the best products, and
providers need technologies that facilitate their work.
In this context, the use of combination vaccines minimizes the need for increased storage capacity and
reduces vaccination operational costs (38). Thus, the alternative of using
a fully liquid hexavalent
acellular combined vaccine in the current Peruvian vaccination schedule could enhance cold chain
performance nationwide, with a stronger focus on the country's most inaccessible areas (39).
Peru's cold chain has made significant strides, but there are gaps that must be addressed effectively,
emphasizing the importance of up-to-date, integrated information enabling appropriate management that
positively impacts the introduction of new vaccines, vaccination during pandemics or other contingency
situations, and health emergencies.
Authorship contributions:
Authors A. Gutiérrez, MA Mendoza, PA Rodríguez, and T. Sarazu contributed to the study
conception. Data collection was performed by MA Mendoza, and data analysis was conducted by
A. Gutiérrez and MA Mendoza. The manuscript was prepared by A. Gutiérrez and MA Mendoza. All
authors have read and approved the final manuscript: PA. Rodríguez, T. Sarazu, A. Gutiérrez,
and MA. Mendoza.
Financing:
This study was financially supported by Sanofi Vaccines.
Declaration of conflict of interest:
PA. Rodríguez and T. Sarazu are employees of Sanofi Vaccines; the other authors declare no
conflicts of interest.
Recevied:
December 19, 2023
Approved:
April 11, 2024
Correspondence author:
Alfonso Gutiérrez Aguado
Address:
Av. Alfredo Mendiola 5210, Los Olivos 15306, Lima-Perú.
Phone:
(01) 2132760
E-mail:
agutierreza@continental.edu.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES