CLINICAL CASE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2023 - Universidad Ricardo Palma10.25176/RFMH.v23i4.6202
CASE REPORT ABOUT IMMUNOTHERAPY IN MANAGEMENT OF METASTATIC RENAL CANCER: TREATMENT WITH CHECKPOINT PD-L1 AND/OR ANTI-CTLA-4 INHIBITORS
LA INMUNOTERAPIA EN EL MANEJO DEL CÁNCER RENAL METASTÁSICO: EN TRATAMIENTO CON INHIBIDORES DE CHECKPOINT PD-L1 Y/O ANTI-CTLA-4. A PROPÓSITO DE UN CASO.
Robert Malpartida Palomino
 1,2,a
1,2,a
Jesús Miguel Malpartida Palomino
 3,b
3,b
Joseph Matos Malpartida
 4,5,c
4,5,c
1 Department of Medical Oncology Service, Hospital Militar Central, Lima-Peru.
2 IAFAS Peru's Army.
3 Pediatric Ophthalmology Service National Institute of Children's Health of San Borja, Lima-Peru.
4 Universidad Peruana de Ciencias Aplicadas, Lima, Perú.
5 Emergency Hospital Ate -Vitarte.
a Medical Oncologist. Program Director ONCOEP (Programa Oncológico del Ejercito del Perú).
b Ophthalmologist. Jefe del Servicio de Oftalmología Pediátrica INSNSB.
c Medical student.
ABSTRACT
Approximately 15 percent of renal cell carcinoma (RCC) present as locally advanced or metastatic, where surgery is not curative. The prognosis of patients with advanced or metastatic RCC can vary from a few months to a few years, depending on the clinical, pathological, and radiological characteristics of the disease. The advent of the use of anti-monoclonal-based immunotherapy as checkpoint inhibitors in initial systemic therapy for advanced clear cell RCC. We will present a clinical case where immunotherapy plays a role through the use of anti-monoclonal drugs to treat a male patient with metastatic kidney disease who presents a clinical response after surgical treatment and immunotherapy for 2 years without evidence of oncological disease to date.
Keywords: Kidney Cancer; Neoplasm Metastasis; Immunotherapy (Source: MeSH – NLM)
RESUMEN
El carcinoma de células renales (CCR) aproximadamente el 15 por ciento se presentan como localmente avanzado o metastásico, donde la cirugía no es curativa. El pronóstico de los pacientes con CCR avanzado o metastásico puede variar desde unos meses hasta algunos años, según las características clínicas, patológicas, radiológicas de la enfermedad. El advenimiento del uso de anti monoclonales basado en la inmunoterapia como inhibidores de puntos de control en la terapia sistémica inicial para el CCR avanzado de células claras. Presentaremos un caso clínico donde la inmunoterapia juega un rol mediante el uso de anti monoclonales para el abordaje de un paciente varón con enfermedad renal metastásica que presenta respuesta clínica después del tratamiento quirúrgico e inmunoterapia por 2 años sin evidencia de enfermedad oncológica hasta la actualidad.
Palabras clave: Neoplasias Renales; Metástasis de la Neoplasia; Inmunoterapia (Fuente: DeCS – BIREME)
Kidney cancer represents 3.8% of all new cancers, with a mean age of diagnosis of 64 years. According to the Global Cancer Observatory 2020 (GLOBOCAN), worldwide there is an incidence of 431,828 new cases, and a mortality of 1,790,368 cases. Likewise, in Peru, according to GLOBOCAN 2020, it is the 11th most common neoplasia (with an estimated prevalence of 4,466 cases), with an incidence of 1,924 new cases and a mortality of 770 cases, as well as a standardized rate by age and sex of 5.7 per 100,000 inhabitants (1). At the local level, according to the Cancer Registry of Metropolitan Lima (2010 - 2012), kidney cancer ranks twelfth in incidence (930 new cases in men and 491 in women, respectively) (2). According to statistics from the Department of Epidemiology and Cancer Statistics of the National Institute of Neoplastic Diseases (INEN), in the period 2000-2017 a total of 3,663 new cases of kidney cancer have been reported (3).
Several histological subtypes with different genetics and clinical behaviors are included in kidney cancer; the most common is clear cell cancer (70%), the papillary renal cell carcinoma (PRCC) (10 to 15%) and chromophobe (5%). The rest corresponds to other histology. Renal medullary carcinoma is rare (less than 0.5%) and very aggressive (4, 5). The prognostic factors are the clinical stage (6, 7), the histological grade, the local extension of the tumor, nodal disease, and evidence of metastatic disease at diagnosis (8, 10). Suspicion begins with the discovery of a renal mass by ultrasound or by tomography. Many times, the discovery is incidental. The clinical findings of hematuria, mass, and flank pain (classic triad) are becoming less common. Additionally, some systemic symptoms of metastatic disease may present as lymphadenopathy, bone pain, weight loss, among others (11, 12). The treatment of kidney cancer is multidisciplinary and its choice will depend on the stage of the disease, the histological type, the patient's internal factors and, in the case of metastasis, the risk classification of metastatic kidney cancer.
This condition derives into a surgical solution, and although modern treatments are currently available such as targeted therapies with tyrosine kinase inhibitors (TKI), anti-VEGF antibodies, mTOR inhibitors, even immunotherapy (inhibitors of checkpoint PD-L1 and/or anti-CTLA-4), these can give durable clinical responses, but will not be able to cure without complete surgical removal (13). Immune checkpoint inhibitors, cytotoxic T lymphocyte antigen 4 (CTLA-4) and, especially, anti-programmed cell death receptor-1 (PD-1) or its ligand (PD-L1) are predictive biomarkers of clinical response that allow correct selection of patients (14). Immunotherapy in kidney cancer aims to control the characteristics of neoplastic cells, driver mutations, the tumor microenvironment, and the host immune response (15, 16). The oncological entity of metastatic kidney cancer disease is addressed with monoclonal antibodies treatment with immunotherapy combined with clinical response.
54-year-old male patient with official occupation of the Peruvian Army in the rank of active colonel. From the city of Lima with no significant personal history. Family History: Father died of pulmonary fibrosis. The patient does not present first-degree relatives with cancer.
The patient has a history of intermittent cough for approximately 1 year, which worsened in the last month, associated with progressive asthenia. Evidenced anemia in another institution (Hemoglobin: 10.2 g/dL) for which he was evaluated by the Hematology Service of the Central Military Hospital in September 2018. In the debut with a score of 01 on the activity scale of Eastern Cooperative Oncology Group (ECOG).
Patient's laboratory tests show Hemoglobin: 8.4 g/dL, Leukocytes: 13500/mm3, Neutrophils: 10665, Ab: 0%, Platelets: 714000 /mm3, Ferritin: 1039 ng/mL, Glucose: 91 mg/dL, Urea: 24 mg/dL, Creatinine: 0.84 mg/dL, Protein: 8.0 g/dL, Albumin: 3.6 g/dL, Calcium: 8.5 mg/dL, DHL: 215 U/L.
In the Multislice spiral computed tomography (MSCT) of the chest without contrast (08/28/2019) observed in the left upper lobe (LUL) a pulmonary nodule with lobulated edges, 27 x 17 mm, due to probable secondary nature. Incidentally, it is partially appreciated to have a right kidney tumor. Subsequently, MSCT with contrast is performed (09/27/2018) where a right renal mass is evident at the level of the upper pole of 93 x 87 mm with poor interface with adjacent subhepatic border and with the vena cava, with pathological contrast uptake (figure 01). Rest (-) neoplasia, MSCT Brain: (-) neoplasia.
The surgical approach performed was a right laparoscopic nephrectomy (25/10/2018); the result of the anatomopathological study was a clear cell renal carcinoma. Tumor size: 8.2 x 7 cm, nuclear grade (Fuhrman): grade 3, growth pattern: solid and trabecular, invasion: limited to the kidney, perineural lymphovascular invasion: (-), capsular invasion: (-), perirenal fat: (-), renal vein: (-), renal pelvis and ureter: (-). Metastasectomy is scheduled through surgical management by resection of the left pulmonary nodule (19/11/2018) where, macroscopically: a tissue fragment measuring 6 x 2.7 x 1.5 cm is received, with a whitish nodule of 1.5 x 1.3 cm and, microscopically: metastatic clear cell carcinoma in lung with tumor vascular embolism; surgical margin compromised with the neoplasm.
The post-surgical evaluation, conducted by the Oncohematology service of the Central Military Hospital, presented the following laboratory results (04/12/2018): hemoglobin: 12.2 g/dL, leukocytes: 7100 /mm3, neutrophils: 4690 Ab: 0% Platelets: 400000, urea: 38 mg/dL, creatinine: 1.24 mg/dL, GFR: 95.3 mL/min, calcium: 9.7 mg/dL, proteins: 7.0 g/dL, albumin: 4.2 g/dL.
The post-surgical imaging studies, MSCT – TAP (thorax abdomen pelvis) c/c of the chest (07/12/2019): Showed post-surgical changes in the anterior segment of the left upper lobe with a pseudonodular image of irregular edges measuring 42 x 21 mm that captures contrast medium in the periphery and suggests close follow-up. Abdomen: Absence of right kidney + small subcapsular hepatic collection and at the level of the renal surgical bed (figure 2).
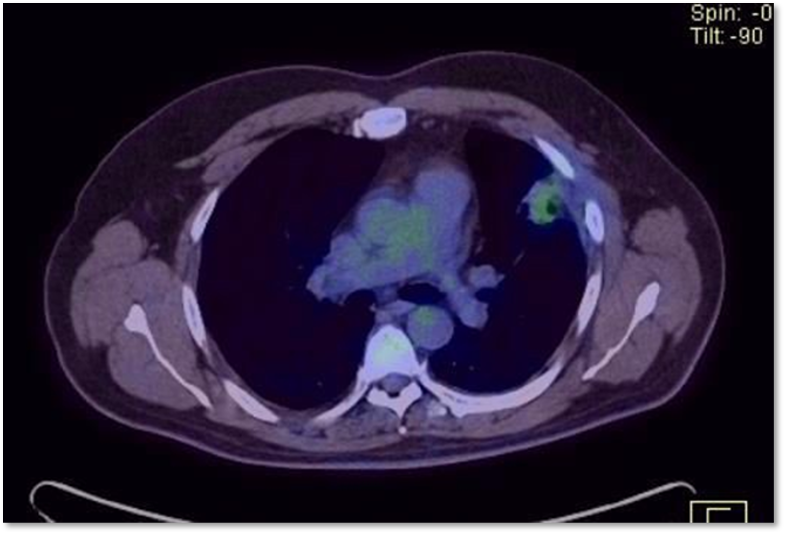
Post-Surgical PET/CT was performed (01/02/2019) where it was presented in the surgical bed and a solid pseudonodular lesion with poorly defined edges measuring 3.7 x 2.2 cm was observed with ill-defined edges measuring 3.7 x 2.2 cm, with increased metabolism with SUV max of 3.1 that persists in late control with Max SUV of 3.7, associated with two nonspecific subcentimeter nodules due to their little size; findings highly suspicious of neoformative disease active (Figure 03). When this evidence is presented, it is classified as Kidney Cancer. Intermediate/poor risk pulmonary metastatic clear cell plus disease residual post pulmonary metastasectomy.
Systemic treatment begins according to the NCCN 2019 clinical guideline under the scheme based on immunotherapy Nivolumab + Ipilimumab (anti-monoclonals). In the 1st Illness Reevaluation (04/08/2019). Post 4 cycles of Nivolumab + Ipilimumab. MSCT imaging study Thorax c/c: Fibrotic parenchymal band in lobe upper left with slight pleural thickening, compatible with sequelae inflammatory. MSCT Abdomen c/c: Sequela of Right Nephrectomy.
The patient presents the following transaminase results where an increase to grade 2 and 3, generating toxicity with the risk of interrupting the treatment with immunotherapy (table 1).
|
|
Basal |
Post |
Post |
Post |
Post |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TGO (UI/L) |
21.0 |
17.0 |
15.5 |
37.1 |
163 (G°2) |
||||||||||||||||||
|
TGP (UI/L) |
18.2 |
12.8 |
11.3 |
44.1 (G°1) |
337.7 (G°3) |
Source: self-made
Due to toxicity in 2nd Disease Reevaluation (08/01/2019). Post 6 cycles of Nivolumab monotherapy. Imaging studies were negative for neoplasia. MSCT Thorax c/c: Fibrous tracts in the left upper lobe with a residual appearance. MSCT Abdomen c/c: Right kidney absent. Rest (-) Neoplasia. (figure 04).
Patient continues his treatment until he reaches the two years established by the American NCCN clinical guideline where there is no evidence of disease and is currently maintained in control without evidence of disease.
DISCUSSION
In this case, the therapeutic decision was based on observation, and tomographic monitoring was conducted over a period of three months. Firstly, the evaluation of a study was continued by PET/CT which revealed secondary disease; a new thoracic resection surgery was performed, and finally systemic treatment for metastatic disease was started. The management of oligometastatic disease in kidney cancer should be personalized and multidisciplinary. Different treatments such as systemic therapy, surgery and stereotactic body radiosurgery (SBRT) are options for the management of this neoplasia.
Patients with oligometastatic disease have a small number of lesions with slow growth kinetics; active observation is an option reasonable, but oligometastatic disease. Cytoreductive nephrectomy associated with immunotherapy is mainly retrospective and derived from large institutional databases, it is highlighted that patient selection is crucial. Cytoreductive nephrectomy should be reserved for patients with no more than one risk factor, those who require palliation of local symptoms, and for those with stable low-volume disease or complete response after exposure to systemic therapy.
It has been considered whether PET/CT is useful in evaluating renal cancer, but there is no formal recommendation for its use in international guidelines. PET/CT is not effective in evaluating the primary renal mass (sensitivity less than 60%). A negative result from PET/CT does not necessarily indicate the absence of malignancy, except in cases of papillary carcinoma or sarcomatoid neoplasms. For evaluating metastatic disease, CT, MRI, and GGO are the standard studies to rely on.
In some cases, when there is a suspicious lesion, a PET scan can confirm whether it's metastatic or not. A study by Rodríguez Martínez de Llano et al showed that in 58 patients, 43% of PET/CT scans resulted in a change in the management of the patient with RCC metastatic (17). Some studies have also evaluated the role of PET and the variation of SUVmax in the response to TKI. Treatment is typically based on International Metastatic Renal Cell Carcinoma Database Consortium criteria, and for most patients with advanced clear cell RCC, systemic therapy is initiated immediately when there is unresectable disease, either metastatic or locally advanced.
The decision to start treating a patient systemically and the choice of medication depends on their symptoms, any pre-existing medical conditions, and the level of risk posed by the tumor. Nivolumab plus ipilimumab has been approved by the FDA for patients with advanced intermediate or high risk RCC who have not received prior treatment. Recent studies have shown that combined immunotherapy treatments have led to a shift towards initial systemic therapy, with the consideration of cytoreductive nephrectomies in responding patients in both early and late phases. In this particular case, the patient has a favorable disease risk, but experienced toxicity due to elevated transaminases. Therefore, the treatment will continue with single agent Nivolumab until completing both years of the treatment. The patient currently shows no signs of oncological disease and has an adequate performance status (ECOG: 0).
Authorship contributions:
RMP, JMMP, JAMM have participated in the conception of the article, data collection, writing and approval of the final version.
Financing:
There is no type of financing.
Declaration of conflict of interest:
The authors declare that they have no conflict of interest.
Recevied:
December 20, 2023
Approved:
December 26, 2023
Correspondence author:
Robert Malpartida Palomino.
Address:
Condominio Golf Los Andes EDF 06 Department 101, 1st. Etapa Naña - Chosica Lima-Peru.
Phone:
981 236 407
E-mail:
robertmp7@hotmail.com
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES