REVIEW ARTICLE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2023 - Universidad Ricardo Palma10.25176/RFMH.v23i4.6206
MURINE MODELS FOR THE STUDY OF TRIPLE NEGATIVE BREAST CANCER
MODELOS MURINOS PARA EL ESTUDIO DE CÁNCER DE MAMA TRIPLE NEGATIVO
Yudith Cauna Orocollo
 1,a
1,a
Ariana Alessandra Córdova Salazar
 1,b
1,b
Jhanina Campos Tineo
 1,a
1,a
1 Instituto de Investigaciones en Ciencias Biomédicas, Universidad Ricardo Palma. Lima, Perú
a Magister en Bioquímica y Biología Molecular
b Bachiller en Biología
ABSTRACT
The study of different variables, such as pathogenesis, inflammatory profile, identification of therapeutic targets, efficacy of treatments in murine models has proven to be one of the most practical for the preclinical study of triple negative breast cancer (TNBC), the most aggressive subtype of cancer, with limited application of treatments and low survival rate. However, it must be recognized that there are other minors in which the induction of TNBC is being standardized. This review encompasses the different induction methods that have allowed the development of TNBC and the most relevant therapeutic applications by which murine models with TNBC were developed.
Keywords: murine model, triple negative breast cancer, induction, antitumor therapy. (Source: MESH-NLM)
RESUMEN
El estudio de diferentes variables, como patogénesis, perfil inflamatorio, identificación de blancos terapéuticos, eficacia de tratamientos en modelos murinos ha resultado uno de los más prácticos para el estudio preclínico del cáncer de mama triple negativo (CMTN), el subtipo de cáncer más agresivo, con limitada aplicación de tratamientos y baja tasa de sobrevivencia. Sin embargo, hay que reconocer que existen otros menores en los que se viene estandarizando la inducción del CMTN. En esta revisión se engloban los diferentes métodos de inducción que han permitido el desarrollo de CMTN y las aplicaciones terapéuticas más relevantes por el que se desarrollaron los modelos murinos con CMTN.
Palabras clave: modelo murino, cáncer de mama triple negativo, inducción, terapia antitumoral. (Fuente: DeCS- BIREME)
INTRODUCTION
Breast cancer is the second most common malignant neoplasia in women, with high global incidence and mortality rates (1), and it could exceed 4.4 million patients by the year 2070 (2). According to molecular classification, breast cancer is divided into subtypes based on the expression levels of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2). The molecular subtypes of breast cancer are luminal A (ER+ / PR+ / HER2-), luminal B (ER+ / PR+ / HER2+), HER2+ (ER- / PR- / HER2+), and triple-negative (ER- / PR- / HER2-), the latter having a worse prognosis compared to other subtypes (3). Additionally, it is divided into basal-like and normal-like subtypes, the difference between these two subtypes lies in the expression of CK5, being CK5+ and CK5-, respectively (4).
To accelerate the discovery of therapeutic targets for prospective treatments and breast cancer prevention, animal models are required for preclinical studies (5). Immunocompromised murine models with triple-negative breast cancer (TNBC) have been developed (4, 5), such as BALB/c strain mice and Sprague-Dawley strain rats, which have allowed the evaluation of pharmacokinetics, pathogenesis, inflammatory profile, microbiome, gene expression levels, and determination of effective doses, toxicity of chemical compounds, etc. (6, 9). The objective of this review is to summarize the methods of TNBC induction in murine models, the therapeutic applications evaluated to date, and their advantages and disadvantages for the study of TNBC.
SEARCH STRATEGY
The search strategy for the bibliographic references of this review was meticulously structured, using a combination of keywords and Boolean operators to refine the results. The keywords used in English were: 'triple negative breast cancer', 'murine model', 'rat model', 'cell', 'xenograft', 'allograft', 'chemical cancer inductor', 'ionizing radiation', 'radiotherapy', 'chemotherapy', 'immunotherapy', and 'probiotics', related to 'animal model breast cancer'. These were combined using Boolean operators such as 'AND' and 'OR' to optimize the search. The search was conducted on search engines like PubMed, Google Scholar, and Science Direct. In addition, stringent selection criteria were established for the articles, prioritizing recent studies with high impact and relevance to the topic, thus ensuring that the review was based on the most current and pertinent literature.
METHODS AND DEVELOPMENT OF MURINE MODELS WITH TNBC
There are chemical, physical, and biological methods that promote carcinogenesis and are administered by different routes.
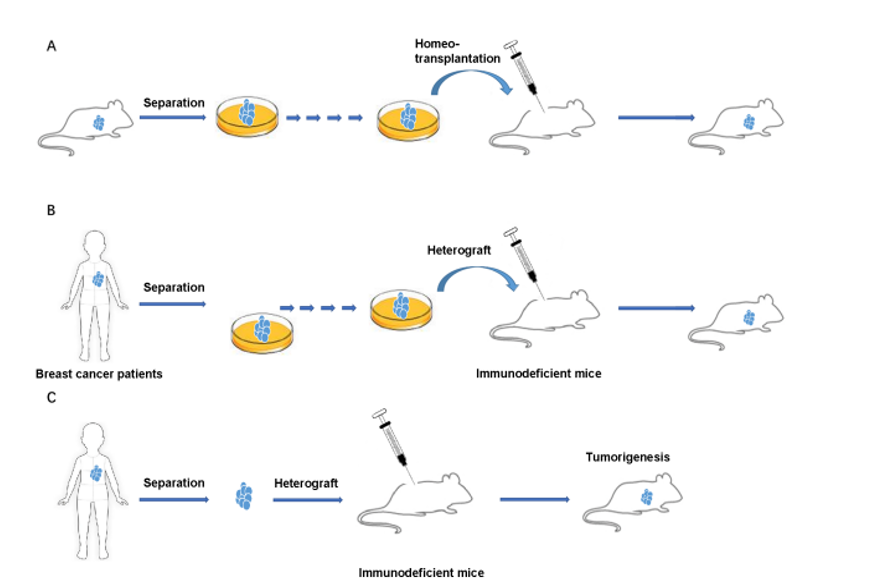
Among the biological induction methods for the development of breast cancer, the transplantation of grafts, cell lines, and tissues from murine models or women with breast cancer was significant in studying the pathogenesis of one of the breast cancer subtypes for the development of personalized therapies. The types of grafts are allografts or homografts, which involve using tissues or cells from one animal and transplanting them into another animal of the same species (Figure 1A), and xenografts or heterografts, which are obtained from humans and transplanted into an animal (Figure 1B, 1C) (9). Although there are two types of grafts, the transplantation of xenografts is of greater importance for preclinical studies, as they involve human grafts.
The success of developing a murine model with breast cancer generally depends on the immunodeficiency of the animal for oncological studies, as the wild type immune response can be an obstacle to tumor growth and metastasis (10). However, the applications of the study, such as the therapeutic composition intended for evaluation, must also be considered (11). Recently, the Sprague Dawley Rag2/Il2rg double-knockout (SRG OncoRat) immunosuppressed model for oncology studies was developed and validated. It showed a reduction in the tissue volume of the spleen and thymus, and consequently, a decrease in circulating T, NK, and B cells (Figure 2) (7).
Biological Induction of TNBC
Transgenic Murine Models Mediated by Viruses
The use of foreign DNA has allowed the development of transgenic murine models for the induction of breast cancer. Lentiviruses promote the overexpression of oncogenes, specifically HER2/ERBB2, PyMT, Wnt, Myc, Ras, and PIK3CA, or the suppression of the expression of these genes in transgenic murine models (9). Mice transgenic for Wnt-1 with extensive ductal hyperplasia is also a murine model for studying the pathology of TNBC (12).
Compared to other methods of breast cancer induction, this method includes high rates of incidence, short latencies, and more reliable results; the disadvantages are long incubation periods, varying times of incidence, and heterogeneous pathological characteristics (9).
Murine Models with Breast Cancer Knockout in Tumor Suppressor Genes
These models were developed by knocking out tumor suppressor genes p53, BRCA1/2, and pTEN in mice using recombinase systems, with 50% of the population susceptible to developing breast cancer (13, 14). Other murine knockout models use inducible systems, such as Cre-loxP for the conditional expression of Brca1, or the Tet-off/Tet-on system for the conditional expression of human PIK3CA¨H1047R, to achieve a high incidence rate (95%) with signs of adenocarcinoma and primary tumor phenotypes over incubation periods of 7 months (15, 16).
Orthotopic Induction Based on Xenografts Derived from TNBC Cell Lines
It is commonly reported that the orthotopic route for cell inoculation in mice is in the mammary fat pad (17, 19). Other more complex orthotopic routes include the intraductal pathway (9, 20, 21), mammary nipple canal (22), right or left flank (23, 24), or heterotopic subdermal (25) or subcutaneous routes (21, 26, 27).
The 4T1 cell line is one of the most commonly used to induce TNBC in the allograft mouse model. Using this cell line, the metastatic TNBC model was developed in different FVB/N and BALB/c mice using the mammary intraductal method, which involves making a cut at the base of the nipple to access the main duct of the mammary glands and inoculating 5 x 10^4 cells in 5 µL (28). Additionally, there are other breast cancer cell lines from genetically modified mice that promote the development of luminal and basal breast cancer and also generate metastases to the lungs and other organs (Table 1) (8).
|
Cell Line |
Origin |
Latency |
Pathology |
Metastasis |
Transfer Site |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
4T1 67NR |
BALB/C |
8-17 d |
luminal |
yes |
lungs |
||||||||||||||||||
|
4T1 4T1.2 |
BALB/C |
8-17 d |
basal |
no |
lungs |
||||||||||||||||||
|
TM40D |
BALB/C |
1 wk |
yes |
lungs |
|||||||||||||||||||
|
D2A1 |
BALB/C |
14 -18 d |
yes |
lungs, corazón |
|||||||||||||||||||
|
EMT6 |
BALB/C |
3 -5 d |
yes |
lungs |
|||||||||||||||||||
|
E0771 |
C57BL/6 |
basal |
yes |
lungs |
|||||||||||||||||||
|
MVT1 |
FVB/N |
luminal |
yes |
lungs |
|||||||||||||||||||
|
6DT1 |
FVB/N |
luminal |
|||||||||||||||||||||
|
M6 |
FVB/N |
44 d |
luminal |
yes |
lungs |
||||||||||||||||||
|
CST |
FVB/N |
20 d |
basal |
||||||||||||||||||||
|
EAC |
Extramural |
yes |
lungs, hígado, corazón, huesos |
d: day, wk: week. Source: adapted from Yang et al. (2017)(8).
Among other murine breast cancer cell lines, one study developed the murine model with TNBC by injecting 10^6 cells from the D2A1 line in 100 µL into the mammary gland, resulting in pulmonary metastasis (29). In another study, 5x10^4 cells in 20 µL of the murine JygMC(A) cell line, with a triple-negative phenotype, were inoculated into the inguinal pad of the mammary fat tissue of athymic mice, observing the early appearance of tumors (30).
Human cell lines isolated from patients with TNBC have also been employed for the development of murine xenograft models, considering the negative expression of ER, PR, and HER2, and the histopathological characteristics generated by each cell line (Table 2); moreover, they have been classified into two subtypes of TNBC, Triple-Negative A or basal-like (TNA) and Triple-Negative B or normal-like (TNB) (31).
|
Cell Line |
ER |
PR |
HER2 |
Tumoral Classification |
Histopathological Characteristics |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
HMT-3522 |
- |
- |
- |
TNA |
benign tumor |
||||||||||||||||||
|
DU-4475 |
- |
- |
- |
TNA |
Invasive Ductal Carcinoma |
||||||||||||||||||
|
HCC-1806 |
- |
- |
- |
TNA |
Squamous Carcinoma |
||||||||||||||||||
|
HCC-70 |
- |
- |
- |
TNA |
Ductal Carcinoma |
||||||||||||||||||
|
MA-11 |
- |
- |
- |
TNA |
Invasive Lobular Carcinoma |
||||||||||||||||||
|
MDA-MB-231 |
- |
- |
- |
TNB |
Adenocarcinoma |
||||||||||||||||||
|
MDA-MB-157 |
- |
- |
- |
TNB |
Medullary Carcinoma |
||||||||||||||||||
|
SUM-149PT |
- |
- |
- |
TNB |
Inflammatory Ductal Carcinoma |
||||||||||||||||||
|
SUM-159PT |
- |
- |
- |
TNB |
Adenocarcinoma |
TNA: Triple Negative A, TNB: Triple Negative B, (-): negative. Source: adapted from Costa et al. (2020)(31).
Among the mentioned human cell lines for the induction of TNBC, MDA-MB-231 stands out as one of the most used. In one study, 10^7 MDA-MB-231 cells in 100µl were subcutaneously inoculated into the right flank of nude mice, resulting in the formation of visible tumors in less than 15 days (23). Nofiele & Cheng employed the same cell line to induce TNBC in healthy 6-week-old immunodeficient female rats and determined that ultrasound is necessary for the appearance of primary tumors (32).
In another study, non-obese diabetic immunodeficient (NOD/SCID) mice were used, into which 3 x 10^6 cells in 100µl were inoculated in the mammary fat pad, observing a slower initial growth of MDA-MB-231 tumors compared to 4T1 tumors (25).
Induction Based on Xenografts Derived from Breast Tissue with TNBC
The development of breast cancer in murine models from xenografts derived from breast tissues of patients with TNBC has been a significant challenge in terms of histocompatibility. For instance, nude mice with a mutation in the Foxn1nu gene, deficient in B lymphocyte development and with increased activity of NK lymphocytes, can tolerate xenografts derived from human cell lines, but not xenografts derived from human tissues (33).
Recently, a murine model of patient-derived xenograft (PDX) was developed from tumor aspirates, which were concentrated and orthotopically transplanted into immunodeficient mice. It was reported that out of 269 xenografts, 62 were successful (34), indicating a very low efficiency of this method for the development of murine models with TNBC.
Induction of TNBC by Chemical Compounds
Carcinogen induction is one of the most practical methods for the development of breast cancer in murine models. The carcinogens commonly used in murines include DMBA (7,12-dimethylbenz[a]anthracene), MCA (3-methylcholanthrene), 1,2,5,6 dibenz[a]anthracene, MNU (N-methyl-N-nitrosourea), 2-acetylamino-fluorene, 3,4-benzopyrene, ethylnitrosourea, and butylnitrosourea, which generate B-type adenomas and adenocarcinomas (9, 35, 36). DMBA and MNU, which are hormone-dependent, induce breast cancer in rats with ER-alpha-positive type.
On the other hand, ACI, Sprague Dawley, Fisher 344, Inbred S-D rats administered with 17 β-estradiol overexpressed ER and PR, and binding protein 3 (Gata3), making them an ideal model for the study of luminal subtype breast cancer or hormone-dependent breast cancer (9, 21, 37, 38). No information was found on carcinogens used specifically for the development of TNBC.
Induction of TNBC by Ionizing Radiation
There are few studies focused on the specific induction of TNBC by ionizing radiation (IR). The most commonly used type of IR were X-rays for the induction of breast cancer under a sublethal dose, 0.2 Gy, in Sprague Dawley rats and BALB/c mice (39). Other types of ionizing radiation (IR), such as 177Lu neutrinos and gamma rays, are also capable of inducing lymphopenia and tumorigenesis in murine models (39, 40).
In another study, it was evidenced that mammary tumors in irradiated rats were primarily hormone-dependent adenocarcinomas and fibroadenomas. However, in 2020, X-rays were used on MDA-MB-231 cells to generate a TNBC xenograft model with characteristics of radioresistance (41).
THERAPEUTIC APPLICATIONS IN MURINE MODELS WITH TNBC
Chemotherapy in Murine Models with TNBC
Chemotherapy is the primary systemic treatment against early or advanced TNBC, however, the cellular heterogeneity found in TNBC patients promoted resistance to this type of therapy (42).
Previously, GEMM mice (BRCA1 mutant breast cancer mice) (BRCA1Co/Co - MMTV-Cre-p53+/− mice) were used to evaluate Cisplatin against TNBC, individually or combined with OMO-1, a selective inhibitor of the epithelial-mesenchymal transition factor (c-MET), which reduced tumor progression (22, 43). In another study, the action of Veliparib and Olaparib as ADP ribose polymerase (PARP) inhibitors was evaluated, and a delay in the development of BRCA1-deficient (TNBC) tumors was observed in a mouse model (44).
On the other hand, chemotherapy may require supplementary compounds to enhance the activity of drugs. In one study, the antitumor effect of doxorubicin/cyclosporine combined with IMMUNEPOTENT CRP, an immunomodulator comprising a mixture of small molecules derived from bovine spleen, was observed in an allograft murine model with TNBC (45). Other chemotherapy regimens act synergistically with immunotherapy. This is the case with α5β1 integrin-marked paclitaxel micellar (ATN-MPTX), ATN protein deposited in micelles, plus nano-STING, an activator of the innate immune pathway STING, resulting in reduced tumor volume and mitigated lung metastasis (46).
Radiotherapy in Murine Models with TNBC
Radiotherapy is a form of treatment based on the use of X-rays or gamma rays to halt cancer development, following criteria of effective dose, exposure time, and localized or complete administration (47).
Hormonal therapies are not useful for TNBC, hence radiotherapy is one of the treatment options against TNBC (40, 48). Various studies have demonstrated its efficacy in reducing the likelihood of locoregional recurrence and increasing patient survival rates. However, these are insufficient, added to the resistance and side effects caused by high doses of IR (47, 49).
In recent years, new radio sensitization targets have been identified, such as alkylphosphocholines overexpressed in malignant cells. Its analogue is 18-(p-iodophenyl) octadecyl phosphocholine, which, when marked with the radioisotope 125I, becomes CLR 125, a radiotherapy agent. It was administered in murine models with subcutaneous and metastatic TNBC xenografts, resulting in the destruction of cancer cells (50).
In one study, the effectiveness of tumor cell destruction based on proton therapy in mice with MDA-MB-231 xenografts was reported (51). In the same group of novel therapies is photobiomodulation (PBM), which, in combination with radiotherapy in BALB/c allograft mice, showed that PBM retained tumor growth, attenuated the negative effects of radiotherapy, and halted metastasis (47). Another radiosensitization method evaluated in allograft mice was based on the use of D-mannose, a sugar that destabilizes the mRNA of BRCA1, RAD50, and MRE11, which inhibited tumor growth (48). Likewise, radiochemotherapy based on 177Lu-NM600, evaluated in an allograft murine model, was effective against TNBC, increasing survival time (40).
Immunotherapy in Murine Models with TNBC
Generally, immunotherapy is based on the use of antigens identified in tumor cells to generate a tumoricidal effect and suppress the production of immunosuppressive molecules, addressing resistance to chemotherapy (8, 42).
One of the immunotherapy methods against TNBC in a heterotopic murine model was the administration of antibodies against aspartic protease cathepsin D (cath-D), overexpressed in breast cancer tumor cells, which inhibited tumor growth and promoted the activation of NK cells and inactivation of M2 macrophages (52). However, the tumor infiltrate also consists of a heterogeneous immune profile that generates immunotherapeutic resistance (36, 53). There are 2 immune subtypes of TNBC, the neutrophil-enriched immunosuppressive subtype (NES), and the macrophage-enriched subtype (MES), both resistant to immune blockade (53).
Due to immune heterogeneity, vaccines based on tumor membrane vesicles from the 4T1 cell line (TMV vaccine) combined with anti-CTLA-4 antibodies have been developed, promoting the activation of T lymphocytes against TNBC in allograft murine models (54). On the other hand, an immunotherapeutic method with high tumoricidal activity based on granulocyte-macrophage colony-stimulating factor (GM-CSF) was developed in an allograft mouse model (55). Xu et al. developed puerarin in nanoemulsion, named nanoPue, which, when administered in murine models with TNBC, reduced the activity of tumor-associated fibroblasts and increased the infiltration of cytotoxic T lymphocytes and M1 macrophages (56).
Probiotic-Based Therapy in Murine Models with TNBC
Due to the high rate of resistance to the aforementioned therapies, alternative complementary approaches effective against TNBC have also been evaluated. In recent years, the importance of probiotics, strains of microorganisms with anti-inflammatory, immunomodulatory, antimetastatic, and antiangiogenic properties, has been recognized, as the gastrointestinal microbiota regulates the adaptive immune system, and its alteration can lead to immunosuppression and increased susceptibility to cancer.
For instance, it was demonstrated that the oral administration of Lactobacillus acidophilus, Lactobacillus plantarum encapsulated in selenium nanoparticles, Lactobacillus helveticus R389, Bifidobacterium longum RAPO combined with anti-PD-1 antibodies, and fermented milk with Lactobacillus casei CRL 431 reduced tumor growth and metastasis, favored the infiltration of CD4+ and CD8+ lymphocytes, and induced apoptosis of tumor cells in the 4T1 allograft murine model (58, 60, 62). Similarly, the oral administration of Bifidobacterium bifidum in the same model reduced the expression of Ki67, a protein associated with cell proliferation, and increased the expression of p53, a tumor suppressor protein (63).
ADVANTAGES AND DISADVANTAGES OF USING MURINE MODELS WITH TNBC
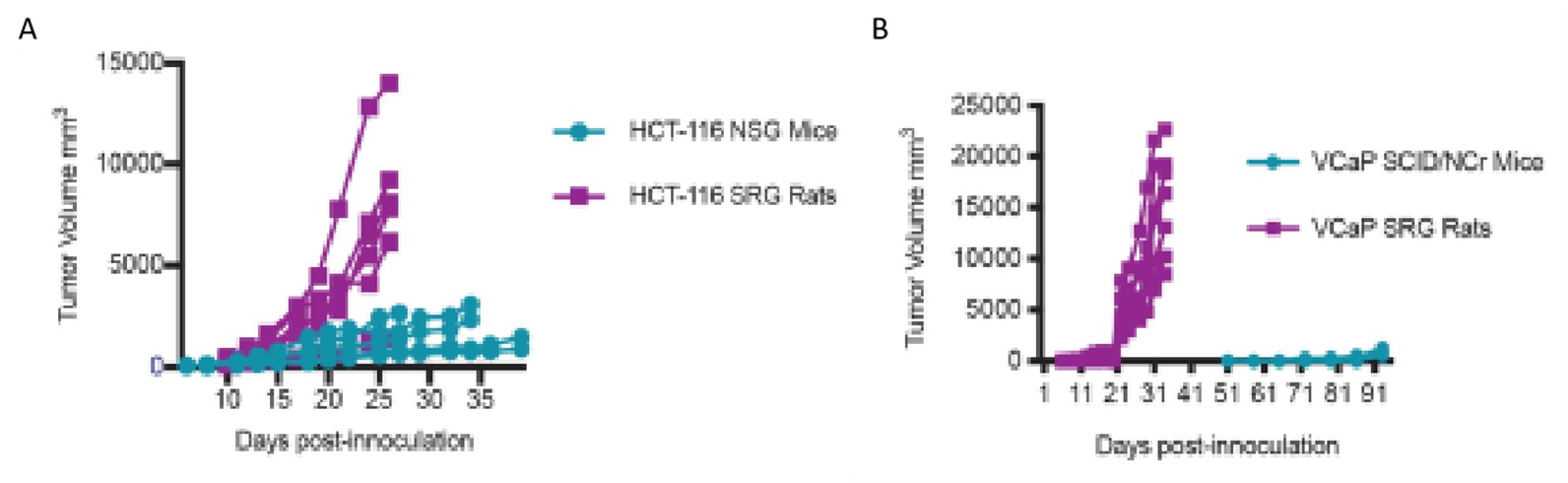
BALB/c mice are the most commonly used murine models in TNBC research studies (9, 18, 32, 64), with NSG (NOD/SCID/γc−/−) mice being the most effective immunosuppressed models for the development of metastasis from breast cancer, compared to nude (athymic) mice (Puchalapalli et al., 2016). On the other hand, Sprague-Dawley rats, also known as Holtzman rats (Rattus norvegicus), have been less used but showed greater advantages due to their larger volume of tissue and fluid biopsies in contrast to NSG and SCID/NCr immunosuppressed mice (Figure 3), greater tumor growth, better capacity for non-invasive imaging, and easier surgical manipulation (7).
One of the disadvantages of using murine models, whether mice or rats, is the demand for extensive vivarium areas and financial resources, as well as specialized human resources for handling these animals. Other less demanding animal models are being developed, smaller in size and more cost-effective, for example, the zebrafish model (65). In this model, it has been noted that the time for cancer development, depending on its stage, can be between 5 to 7 days (larval stage) or weeks to months (immunosuppressed adult stage), as in mice, but using a larger number of cells (10^5 - 10^6 cells) than in zebrafish (65). However, the size of biopsies for histopathology remains a benefit for the use of murine models, even more so in rats (7).
CONCLUSIONS
Thus, animal models, particularly murine models, are relevant in the study of TNBC. The methods developed for the induction of TNBC, using cell lines for allograft models (e.g., mouse 4T1 cell line) and xenograft models (e.g., human MDA-MB-231 cell line), with prior immunosuppression of the animal, have been successfully developed for the evaluation of the pathology and the proposal of therapies against TNBC. As previously reported, TNBC has not been successfully induced by chemical compounds, and there are very few studies reporting the induction of TNBC by ionizing radiation.
Among the therapeutic applications evaluated in murine models with TNBC, innovative treatments have been proposed to address the high rate of resistance to chemotherapy, mediated by ionizing radiation, immunotherapy, and probiotic consumption, with the regulation of the microbiome being a complementary mechanism to achieve a greater antitumor effect.
Autorship contributions:
The authors participated in the genesis of the idea, project design, data collection and
interpretation, analysis of results and preparation of the manuscript of this research work.
Funding:
Self-funded.
Conflicts of interest:
The authors declare that they have no conflict of interest.
Received:
October 10, 2023
Approved:
December 20, 2023
Correspondence:
Yudith Cauna - Orocollo.
Address:
Jr. Enrique Barreda 314, Lima - Perú.
Phone:
(+51) 949610400
E-mail:
yudith.cauna@urp.edu.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES