REVIEW ARTICLE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2024 - Universidad Ricardo Palma10.25176/RFMH.v24i2.6518
GLOBAL APPROACH TO THE PATIENT WITH DIABETIC FOOT: A REVIEW
ABORDAJE GLOBAL DEL PACIENTE CON PIE DIABÉTICO: UNA REVISIÓN
Jordi Viadé-Julià
 1,a,
John Longa-López
1,a,
John Longa-López
 1,2,a,
María Nicolás-Piera
1,2,a,
María Nicolás-Piera
 3,a,
Miquel Sabriá-Leal
3,a,
Miquel Sabriá-Leal
 1,4,a
1,4,a
Melcior Lladó-Vidal
 1,5,a,
Fernando José Muñoz-De La Calle
1,5,a,
Fernando José Muñoz-De La Calle
 6,a,
Xavier Madirolas-Alonso
6,a,
Xavier Madirolas-Alonso
 1,4,a
1,4,a
Marc Sirvent-González
 7,b,
Clàudia Riera-Hernández
7,b,
Clàudia Riera-Hernández
 8,a,
Cristian Carrasco-López
8,a,
Cristian Carrasco-López
 8,a,
Ricard Pérez-Andrés
8,a,
Ricard Pérez-Andrés
 8,a,
Alfonso Rodríguez-Baeza
8,a,
Alfonso Rodríguez-Baeza
 1,a
1,a
1 Universidad Autónoma de Barcelona. Barcelona, España.
2 Instituto de Investigación en Ciencias Biomédicas, Universidad Ricardo Palma. Lima, Perú.
3 Hospital Universitario Mútua de Terrassa. Barcelona, España.
4 Parc Hospitalari Martí Julià. Girona, España.
5 Hospital Universitario Son Espases. Palma de Mallorca, España.
6 Hospital General de Medellín. Medellín, Colombia.
7 Hospital General de Granollers. Barcelona, España.
8 Hospital Universitario Germans Trias i Pujol. Badalona, España.
ABSTRACT
Diabetic foot is one of the most prevalent and severe complications that can develop in patients with
diabetes mellitus, presenting numerous clinical challenges due to the complexity of the lesions and the
multiple associated comorbidities. Effective resolution of this condition requires coordinated
participation from a multidisciplinary team of specialists, including endocrinologists, surgeons,
orthopedists, and specialized nurses. The objective of this paper is to present the "System for the
Evaluation and Treatment of Diabetic Foot" as an integral and user-friendly tool that facilitates the
management of diabetic foot in various clinical settings, including primary care, hospitals, and
emergency services. This system standardizes evaluation and treatment, enhances communication among
professionals, and has demonstrated, over more than 20 years, a significant reduction in the number of
amputations and an improvement in clinical outcomes for patients.
RESUMEN
El pie diabético es una de las complicaciones más prevalentes y graves que pueden desarrollar los
pacientes con diabetes mellitus, y su manejo presenta numerosos desafíos clínicos debido a la
complejidad de las lesiones y las múltiples comorbilidades asociadas. La resolución efectiva de esta
condición requiere la participación coordinada de un equipo multidisciplinario de especialistas,
incluyendo endocrinólogos, cirujanos, ortopedistas y enfermeras especializadas. El objetivo de este
trabajo es presentar el "Sistema para la Evaluación y Tratamiento del Pie Diabético" como una
herramienta integral y fácil de usar que facilita el manejo del pie diabético en diversos entornos
clínicos, incluyendo la atención primaria, hospitales y servicios de urgencias. Este sistema estandariza
la evaluación y el tratamiento, mejora la comunicación entre profesionales y ha demostrado, a lo largo
de más de 20 años, una significativa reducción en el número de amputaciones y una mejora en los
resultados clínicos de los pacientes.
INTRODUCTION
The foot, specifically the sole, is the body region that contacts the ground in both standing and walking due to our bipedal posture. A significant aspect of ambulation is largely the cultural evolution of our species. This important function allows us different modes of movement such as walking, running, and practicing sports, among others. For this, we have a series of proprioceptive and nociceptive receptors. These receptors allow us to interact with the ground and maintain balance in various modes of ambulation through what is known as Hilton's Law(1).
There are evident homologies in the embryonic development(2) of the autopod observed around the sixth post-fertilization week of the hand and foot (hand and foot plates) and in the general anatomical organization of both structures (similarities between skeletal elements, intrinsic and extrinsic muscles, vessels, and nerves). However, the different functions determine the morphological adaptations observed in each.
DIABETIC FOOT
Diabetic Foot (DF) is understood as the “presence of signs, symptoms, or ulcers on the foot as a consequence of chronic diabetes complications”(3). DF is one of the most prevalent complications in patients with diabetes mellitus (DM)(4). In patients with DM, the risk of developing a foot ulcer can reach up to 25%(5).
When a patient with DM develops an ulcer, several factors converge, such as changes in pressure points, deformities, or the use of inappropriate footwear, in addition to the total or partial loss of protective capacity (sensitivity), combined with underlying vegetative and vascular disorders. Consequently, the epidermal layer breaks down, and a lesion appears that can progress to deeper parts and reach the bone (3, 5), endangering the limb and even the patient’s life.
Managing foot lesions is usually complex and requires coordinated participation from different professionals. Studies have shown that a multidisciplinary approach is the most effective way to treat these patients and reduce the number of amputations (6-8). Therefore, we propose our “Diabetic Foot Evaluation and Treatment System” to facilitate this approach (9).
This review aims to provide a comprehensive overview of DF management, highlighting the importance of a multidisciplinary approach and presenting an easy-to-use evaluation and treatment system applicable in any work setting, from primary care to hospitals or emergency services. Additionally, limitations and possible biases in the reviewed studies are discussed to provide a more balanced and critical view of the literature.
EPIDEMIOLOGY OF DIABETIC NEUROPATHY
The prevalence of diabetic neuropathy (DN) varies according to the series consulted, depending on the diagnostic methodology, criteria used, and disease duration in the studied population, making it difficult to compare prevalence rates across different regions. In this context, Pirart J. evaluated 4,400 patients with diabetes mellitus (DM) over 25 years of follow-up (10-13). In this study, neuropathy was defined as a decrease in foot sensitivity and a decrease or absence of the Achilles reflex. The onset of neuropathy positively correlated with the duration of DM, and at 25 years, 50% of patients had developed neuropathy.
In Spain, Mundet et al. evaluated the prevalence and incidence of macro and microvascular complications over ten years of follow-up in a prospective population-based study that included 317 patients with type 2 DM, finding a DN prevalence of 26.8% [19.3-30.2] at the end of the study (14). In Latin America and the Caribbean, a systematic review and meta-analysis of 29 studies from eight countries in the region reported an estimated DN prevalence of 46.5% (95% CI: 38.0-55.0) with significant heterogeneity (I² = 98.2%; p < 0.01), finding an increasing trend in cumulative DN prevalence over time. In this same study, four investigations in Peru with a sample size of 874 patients reported a DN prevalence of 52% (15).
However, it is important to note that up to 50% of patients with DN may be asymptomatic, increasing the gap of underdiagnosis of this complication. According to Longa J. in his study "Attitudes of Physicians Towards the Management of Diabetic Neuropathy in Public and Private Health Facilities, 2023", of 143 physicians surveyed, 80.5% reported relying only on symptoms and signs referred by the patient to diagnose DN (16).
The high rate of DN produces substantial morbidity, including disability generated by painful neuropathic symptoms and the underlying neurological deficit, which have a significant impact on these patients' quality of life and result in manifestations such as ataxia, weakness, falls, fractures, lacerations, cranial trauma, recurrent lower limb infections, ulcerations, and subsequent amputations. Patients diagnosed with diabetic foot (DF) occupy more hospital beds than those with other diabetic complications (17). The cumulative risk of lower limb amputation in one study was 11% 25 years after the DM diagnosis (18).
Risk factors for DN studied vary according to the strength of association. Those with a very strong association include diabetes duration, hyperglycemia, and age. Those with a strong association include prediabetes, height, hypertension, obesity, metabolic syndrome, oxidative stress, vitamin D deficiency, genetic factors, subclinical inflammation, and low physical activity. Those with a moderate association include glycemic variability, dyslipidemia, smoking, insulin resistance, alcohol consumption, hypoinsulinemia, platelet activation, and growth factor depletion (19).
DIAGNOSIS OF DIABETIC NEUROPATHY
Screening for diabetic neuropathy (DN) should be performed in patients with type 2 diabetes mellitus (DM2) at the time of diagnosis. In patients with type 1 diabetes mellitus (DM1), screening should be performed five years after diagnosis. Additionally, prediabetic patients should be included in this screening if they present neuropathic symptoms. If the initial examination is negative, it should be repeated annually (20).
The diagnosis of DN is based on three fundamental pillars: evaluation of symptoms, signs, and, in some cases, the performance of neurophysiological and/or morphometric tests. Symptoms can be classified as positive or negative, depending on whether there is a gain or loss of function, resulting from the maladaptive response to somatosensory nervous system damage or pathology. The first group of symptoms (positive) includes paresthesias, spontaneous pain (burning, searing, stabbing, etc.), or evoked pain (hyperalgesia or allodynia). The second group (negative) can include sensory deficits such as hypoesthesia, anesthesia, hypoalgesia, or analgesia. These clinical manifestations can coexist or alternate throughout the natural history of DN.
Systematic evaluation of symptoms can be conducted through validated questionnaires, such as the Michigan Neuropathy Screening Instrument (MNSI), the Utah Early Neuropathy Scale (UENS), the United Kingdom Screening Test, and the Total Symptom Score (TSS). From a pathophysiological perspective, the involvement of thin nerve fibers (C or Aδ), characterized by being unmyelinated or finely myelinated, clinically manifests as burning pain, electric shocks, or stabbing pain. Autonomic symptoms can also occur since these fibers are responsible for thermoalgesic sensitivity and autonomic function.
As for the thick fibers (Aα or Aα/β), their involvement can cause numbness-type pain, a feeling of walking on cotton, difficulty performing fine tasks like turning book pages or buttoning a shirt, and balance or musculoskeletal trophism alterations, occasionally leading to an inability to stand on the tip of the toes or heels. These myelinated fibers control muscle function and tactile, vibratory, and proprioceptive sensitivity.
In physical examination, signs such as dry skin, fissures, plantar hyperkeratosis, ulcers, overlapping or rigid toes, hammer or claw toes, deformities, bony prominences, Charcot neuroarthropathy, and atrophy of the interosseous muscles may be found. Clinical evaluation of the different types of nerve fibers depends on the availability of instrumental resources, and for this purpose, the Semmes-Weinstein 10 g monofilament, the 128 Hz tuning fork, and the reflex hammer can be used to assess thick fibers, while the thermal bar and pinprick are used to assess thin fibers.
It is important to note that none of these tests alone achieve the sensitivity and specificity needed for DN diagnosis, so combining two or more of them is necessary to confirm the diagnosis. Additionally, there is no standardization of the evaluation methodology, which can hinder early neuropathy detection (21).
Neurophysiological and/or morphometric tests are important tools but are limited in use due to their complexity and limited availability in routine medical practice. For thick fiber evaluation, nerve conduction velocity (NCV) studies for Aβ fibers and the DPNCheck, which evaluates Aβ fibers of the sural nerve with good sensitivity (92-95%) compared to NCV, are used. For thin fiber evaluation, skin biopsy can be used to assess C fibers by quantifying intraepidermal nerve fiber density (IENFD), considered the gold standard for this evaluation and capable of detecting early changes. Corneal confocal microscopy (CCM) also allows for evaluating Aδ and C fibers, being a non-invasive, reproducible, fast, and objective method.
Autonomic tests like the Neuropad, Sudoscan, and QSART are useful for assessing C fibers responsible for autonomic functions, including sudomotor functions. While these tools have different sensitivities and specificities, they are useful for this purpose. Quantitative sensory testing (QST) methods allow for the evaluation of both thin (Aδ and C) and thick (Aβ) fibers with good reproducibility (21).
CHARCOT NEUROPATHY
Charcot neuroarthropathy has a multifactorial etiology, with two main theories proposed to explain its development: the neurotraumatic and neurovascular theories. The neurotraumatic theory suggests that poor pain perception in diabetic patients causes repetitive traumas to go undetected, resulting in multiple fractures and collapse of the foot’s bone structure. On the other hand, the neurovascular theory postulates that bone destruction is due to a hypervascular state caused by sympathetic nerve alteration, leading to loss of vasomotor control. This condition causes bone mineral leaching, resulting in osteopenia or osteoporosis, making the tissue more susceptible to low-magnitude fractures.
The inflammatory process is localized and persistent, without systemic repercussions, characterized by increased vascular flow and elevated levels of pro-inflammatory cytokines. This disrupts the RANKL (receptor activator of nuclear factor kappa B ligand) system, increasing the number and activity of osteoclasts, thus enhancing bone resorption. Molecules like calcitonin gene-related peptide, which normally stabilize the capsuloligamentous extracellular matrix, are released less in the context of diabetic foot, promoting an environment of biomechanical instability and generating pressure zones (22). Additionally, healing is compromised due to reduced macrophage activity and angiogenesis, increasing infection risk due to a diminished immune response (23).
The diagnosis of Charcot neuroarthropathy is primarily clinical. Semiological signs include inflammation (phlogosis) and edema. It is crucial to investigate neuropathic changes in the anamnesis and objectively assess temperature differences greater than 2°C compared to the contralateral limb. In advanced stages, inflammatory signs become less noticeable, with bone prominences and foot deformities predominating, especially in the hindfoot, which may include claw toes and dry skin (xerotic) due to loss of moisture (24, 25).
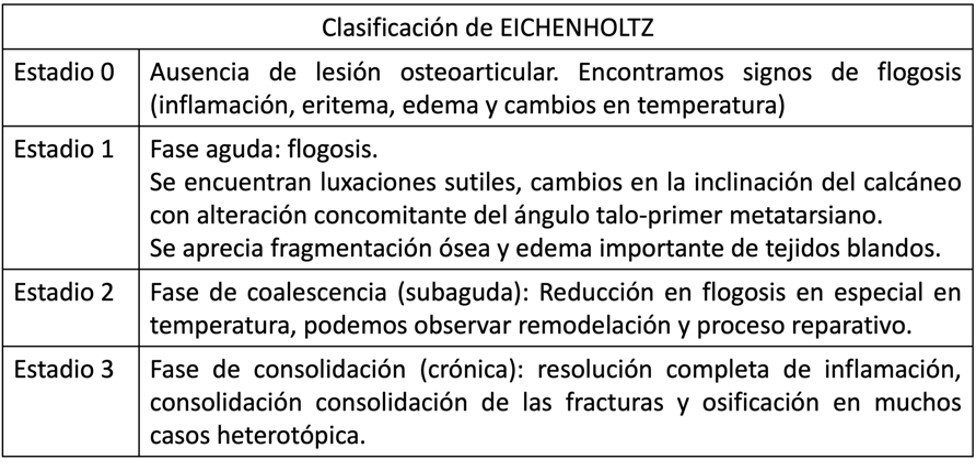
Laboratory tests such as bone-specific alkaline phosphatase and type 1 collagen carboxy-terminal telopeptide levels are useful for quantifying bone resorption in acute phases and decrease as chronicity sets in. Acute phase reactants, such as erythrocyte sedimentation rates below 70 mm/h, indicate a more neuroarthropathic than infectious process. Load-bearing radiographs (posteroanterior and lateral) with oblique views allow for observing deformities and classifying the disease according to the Eichenholtz classification (26). It is important to evaluate deformities in the sagittal plane, considering inclinations at the Chopart level and the pitch of the calcaneal and fifth metatarsal angles.
Table 1. Eichenholtz Classification.
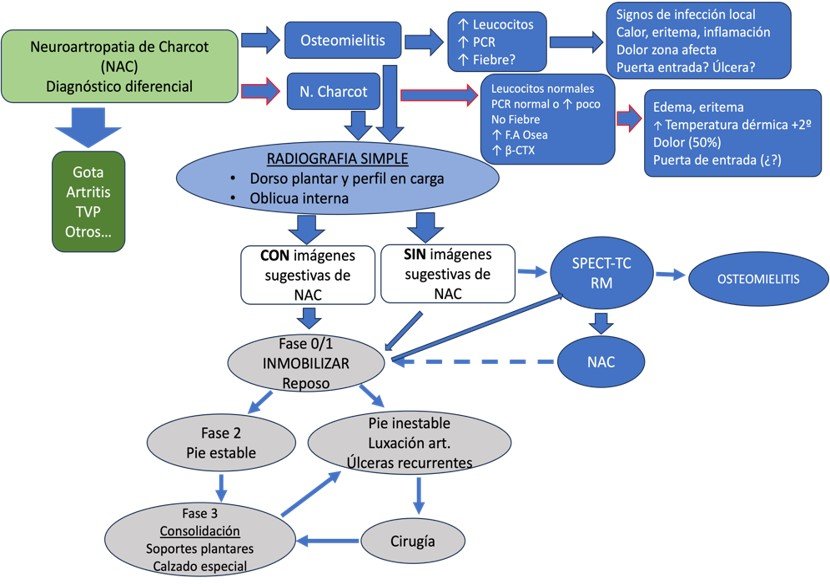
In the acute phase, magnetic resonance imaging (MRI) can reveal subchondral bone marrow edema and microfractures, facilitating the monitoring of the clinical process. Positron emission tomography/computed tomography (PET/CT) shows increased metabolism in the affected regions, allowing for a more sensitive evaluation. Differentiating Charcot arthropathy from an infectious or inflammatory process can be challenging, as they may coexist. Therefore, we propose an algorithm that is part of the diagnosis, evaluation, and treatment system (Illustration 1).
Ilustración 1.Algoritmo diagnóstico de Neuroartropatía de Charcot.
It is recommended to use conservative treatment whenever possible. Offloading is essential and can be achieved through initial total contact casting for six to eight weeks, with changes every two weeks, until the inflammatory state is reduced, allowing for the use of adapted orthopedic footwear. This process generally takes a minimum of six months. Except for the management of metabolic and comorbid conditions in DM patients, there is no evidence of the effectiveness of specific medications for the treatment of Charcot arthropathy (27).
The goal of treatment is to achieve a plantigrade foot with an even distribution of plantar pressures. Maintaining this position can heal up to 50% of neuropathic ulcers without the need for surgical interventions, allowing the patient to return to a functional level similar to the previous one, prevent ulcerations, and reduce long-term medical costs.
Chronic deformities are a significant challenge to correct due to the morphological alteration and the generation of prominent ulcers. A holistic evaluation of the patient is essential, including infection history, previous cultures, treatments, and imaging studies to determine the involved joints. The appropriate fixation material should be planned, and proper cultures taken to use antimicrobials based on the results (3, 27). Interventions may include external fixators, screw and plate fixations, exostectomies, myotendinous transfers, and lengthenings, which can be transitional until achieving a definitive construct (28).
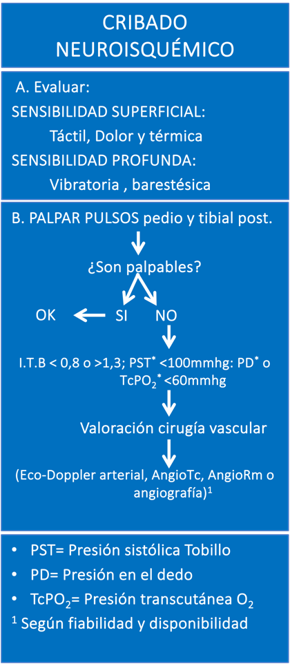
VASCULAR FOOT
Macrovascular disease in patients with DM is characterized by being a diffuse atheromatous process that affects not only the arteries of the lower extremities but also the coronary and carotid arteries. Arterial involvement in peripheral arterial disease (PAD) varies depending on whether the patient has DM. In patients without DM, PAD preferably affects the aortoiliac and femoropopliteal territories. In contrast, in patients with DM, PAD more frequently affects the tibial arteries, that is, the arteries located below the knee. Interestingly, the arteries of the foot are usually preserved, which is relevant when considering treatment options for these patients (3).
Regarding the prevalence of PAD in patients with diabetic foot (DF), current data show that this condition is present in approximately half of the patients. The presence of PAD in patients with DF increases the risk of ulcer infection and hinders its healing (29). This is due, at least in part, to the difficulty of nutrients and oxygen reaching the tissue and the poor penetration of antibiotics in the infected tissue.
The treatment of ischemia in patients with DF remains suboptimal according to data from the Eurodiale study. The main objective of this study, which involved 14 hospital centers, was to analyze the characteristics of 1,229 patients with DM who presented a foot ulcer. Only 40% of patients with severe ischemia underwent angiography and only 43% of patients with critical ischemia underwent a revascularization procedure (30).
Ilustración 2. Algoritmo de Cribaje Neuroisquémico.
COMPREHENSIVE MANAGEMENT OF DIABETIC PATIENTS WITH FOOT ULCERS
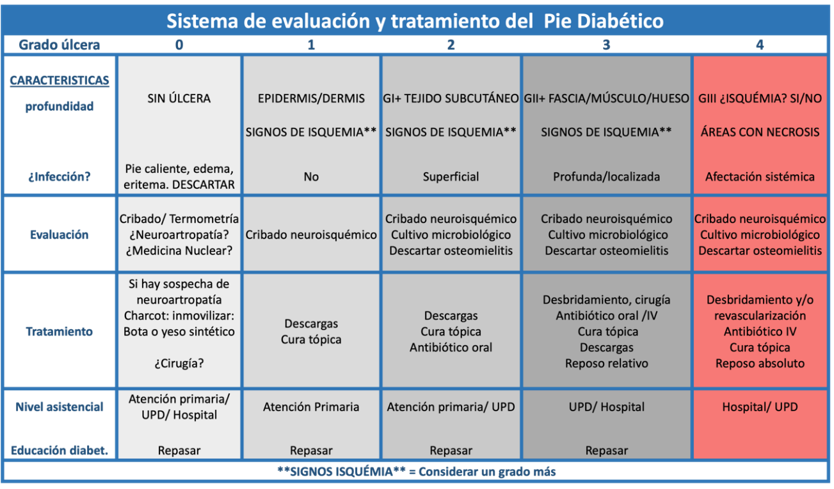
The management of ulcers in diabetic patients is complex due to the need for intervention from multiple professionals. This complexity lies in the coordinated execution of the proposed treatment. To facilitate this multidisciplinary approach, we propose using the "Diabetic Foot Evaluation and Treatment System," published in November 2023 in the journal Foot and Ankle Research.
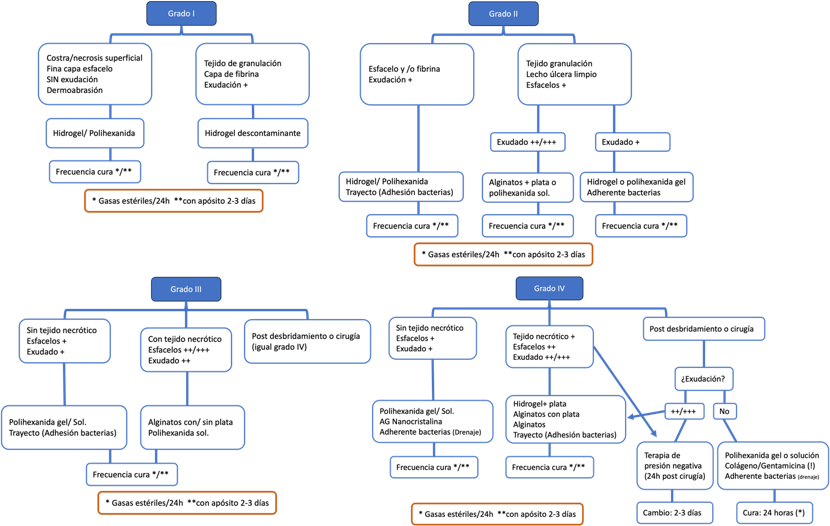
This system (Table 2) consists of a main table that evaluates two fixed variables: the presence of infection and the depth of the ulcer, classifying them into five grades (from 0 to 4). Grade 0 excludes Charcot foot or underlying infections without the presence of an ulcer; grade 1 encompasses superficial ulcers (epidermis/dermis) without signs of infection; grade 2 includes ulcers that reach the subcutaneous tissue with signs of superficial infection; grade 3 comprises deep ulcers that reach the subcutaneous tissue or bone, with signs of deep but localized infection; and grade 4 considers deep ulcers as in grade 3, but also presents critical ischemia, areas of necrosis, and/or systemic involvement. When an ulcer is ischemic in nature, it is considered and treated as an additional grade due to the significantly worsened prognosis.
Ilustración 2. Sistema de evaluación y tratamiento propuesto.
Additionally, the system includes nine supplementary tables or algorithms that address different aspects of DF management: diagnosis of Charcot neuroarthropathy (Illustration 1), neuroischemic screening (Illustration 2), diagnosis of osteomyelitis (Illustration 3), obtaining samples for microbiological culture (Illustration 4), microorganisms to consider (Illustration 5), oral antibiotics (Illustration 6), topical treatment (Illustration 7), offloading systems (Illustration 8), and surgical techniques (Illustration 9). Each of these algorithms provides guidelines for the required examination, differential diagnosis, and the most appropriate treatment for each situation. The implementation of this system allows for a more structured and effective management of ulcers in diabetic patients, optimizing collaboration among the various specialists involved.
INFECTIONS AND ANTIBIOTIC THERAPY IN DIABETIC FOOT
Infection in diabetic patients can be a serious complication. Although most infections are superficial, up to 25% can extend to deeper tissues, even affecting the bone. It is important to remember that an infected foot ulcer precedes 60% of amputations (31). Antibiotic treatment of infections in the diabetic foot requires a deep understanding of the lesion's pathogenesis, with explicit mention of the biofilm and the microorganisms involved. This is essential for selecting the appropriate antibiotic based on the microorganism's sensitivity and various pharmacokinetic characteristics.
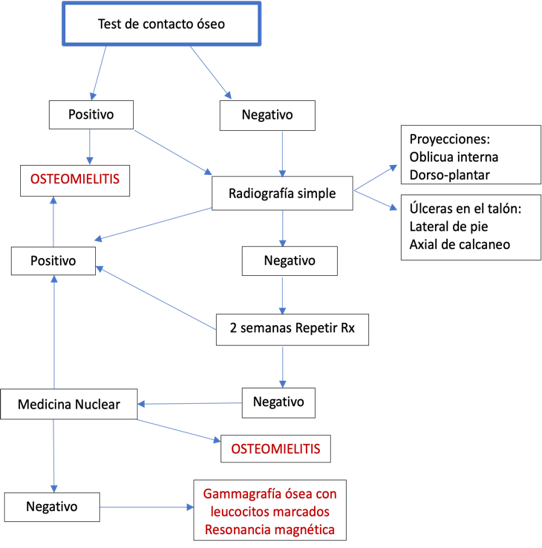
Bone infection, or osteomyelitis, is a common complication of diabetic foot ulcers. To rule out contiguous infection, the bone contact test is performed. Any exposed bone at the base of an ulcer, whether visible or that can be contacted by inserting a sterile blunt-tipped probe, has a high probability of being infected, with a specificity of 83% and a sensitivity of 87% (32). The following algorithm (Illustration 3) assists in the correct interpretation of the bone contact test and the precise diagnosis of osteomyelitis.
Ilustración 2. Test de contacto óseo para diagnóstico de Osteomielitis.
BIOFILM AND MICROBIOLOGICAL CULTURE
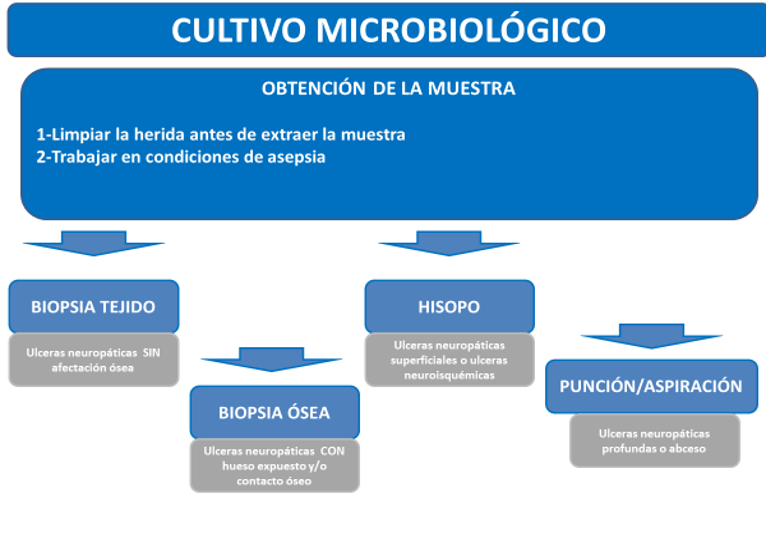
The presence of biofilm in diabetic foot ulcers complicates antibiotic therapy. The biofilm, a biofilm generated by the interaction of cells covering the ulcer and physicochemical and bacterial factors, hinders the penetration and activity of antibiotics, as well as the isolation of the microorganisms responsible for the infection. This latter issue, with poorly collected samples, makes it difficult to differentiate between contamination and infection (33). To etiologically identify the responsible microorganism, it is mandatory to clean and debride the wound before obtaining the sample. This can be obtained by scraping the ulcer with a scalpel, curettage, or surface biopsy. Aspiration of purulent secretions with a sterile needle and syringe can also be useful. All samples should be promptly placed in a sterile container or appropriate medium and sent to the laboratory for Gram staining and aerobic and anaerobic culture (34).
Ilustración 4. Procedimiento para obtención de muestras para cultivo microbiológico.
On the contrary, non-infected ulcers should not be cultured, nor should samples be obtained without prior cleaning or debridement, or by swabbing the wound or purulent secretions. Table 3 presents the microorganisms responsible for diabetic foot infection according to various series (31, 35-37).
| GRAM POSITIVOS | |
|---|---|
| S. aureus | 72 (30) |
| S. coagulasa negativos | 4 (1.7) |
| Enterococcus spp | 8 (3.3) |
| Streptococcus pyogenes | 4 (1.7) |
| GRAM NEGATIVOS | |
| E. coli | 24 (10) |
| Klebsiella pneumoniae | 22 (9.2) |
| Enterobacter spp | 22 (9.2) |
| Proteus spp | |
| Pseudomonas aeruginosa | 28 (11.7) |
| Acinetobacter spp | 12 (5.2) |
| S. maltophilia | 2 (0.8) |
| ANAEROBIOS | 21 (2) |
| OTROS | (>5.2) |
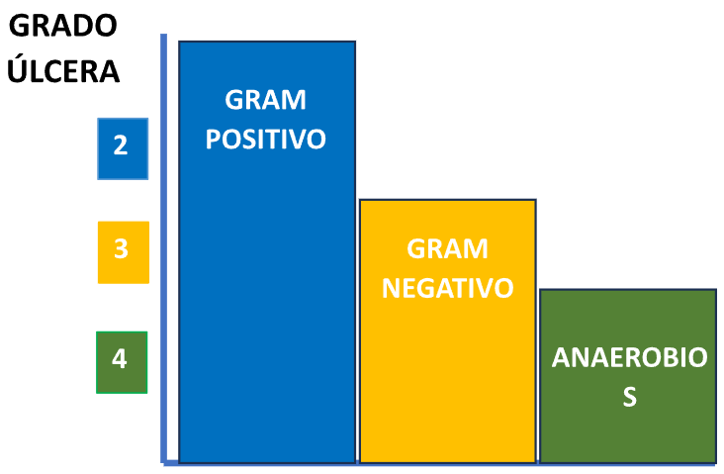
However, depending on the characteristics of the wound, certain microorganisms must be particularly taken into account (Table 4).
| Tipo de herida | Microorganismos responsables de la infección |
|---|---|
| Celulitis o herida cutánea abierta | S. aureus / Streptococcus β hemolíticos |
| Úlcera infectada (NO antibióticos previos) | S. aureus / Streptococcus β hemolíticos |
| Úlcera crónica infectada (antibióticos previos) | S. aureus / Streptococcus β hemolíticos / Enterobacteriaceae |
| Úlcera macerada | Pseudomonas aeruginosa |
| Úlceras de larga duración con antibióticos previos | Cocos gram positivos aerobios, Enterobacterias, Pseudomonas spp y otros BGN no fermentadores |
| Olor fétido, necrosis extensa o gangrena | Flora polimicrobiana: cocos gram positivos, enterobacterias, BGN no fermentadores, anaerobios |
Ilustración 5. Microorganismos a considerar según la gravedad de la úlcera.
As shown in Illustration 5, Gram-positive cocci are usually present in all stages of severity, so it will always be necessary to cover them with appropriate antibiotics. Anaerobes, on the other hand, are observed in severe ulcers and always associated with other microorganisms. In these cases, broad-spectrum antibiotic treatment is required, also considering the possibility of multi-resistant enterobacteria producing extended-spectrum beta-lactamases.
ANTIBIOTIC THERAPY
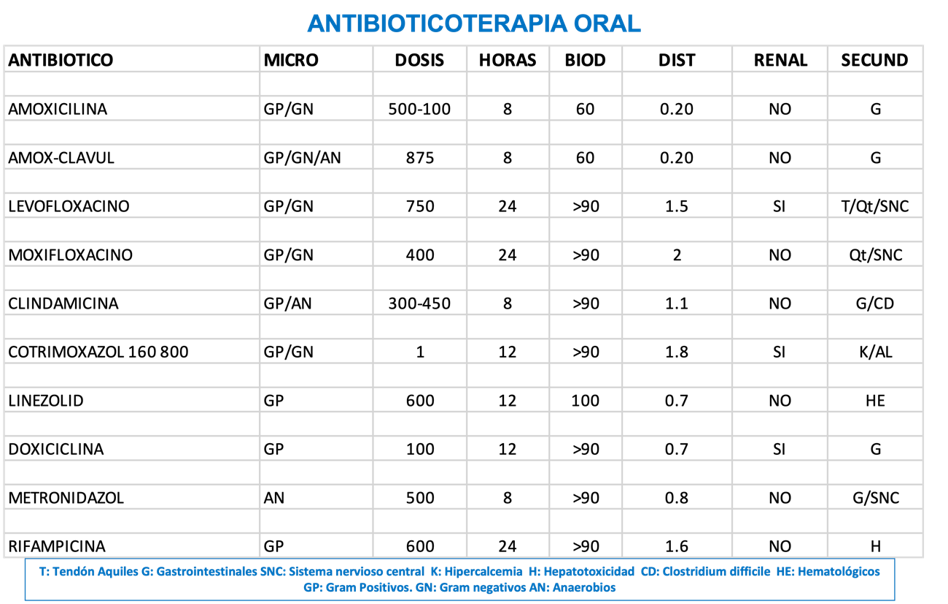
Before administering antibiotics, it is crucial to consider several characteristics inherent to the microorganism and the pharmacokinetics of the antibiotic. This includes the sensitivity of the microorganism to the tested antibiotics according to the antibiogram, the bioavailability of the antibiotic when administered orally, and its volume of distribution (38).
Oral bioavailability is a fundamental characteristic for selecting effective antibiotics via this route. Some antibiotics, such as fluoroquinolones, have a bioavailability close to 100%, while amoxicillin does not reach 70%. Both are useful in diabetic foot, but doses must be adjusted appropriately (39). The volume of distribution is also important, as some antibiotics are preferentially distributed in the vascular compartment, reaching the interstitium and the cellular compartment in low concentrations. In diabetic foot, antibiotics with a high volume of distribution are preferred to ensure they adequately reach the site of infection (40).
Illustration 6 presents a scheme detailing the use and considerations of oral antibiotics in the treatment of infections in diabetic foot. The illustration is divided into several sections addressing critical aspects of antibiotic therapy, such as antibiotic selection, oral bioavailability, and volume of distribution.
Ilustración 6. Antibioticoterapia oral para infecciones del pie diabético.
DURATION OF ANTIBIOTIC TREATMENT
The duration of antibiotic treatment varies according to the severity of the infection. Most ulcers will be sterilized with one to two weeks of oral treatment. In more complicated cases, treatment can start with intravenous antibiotics followed by oral antibiotics to complete two weeks. Suspected osteomyelitis requires treatments of three to four weeks, and after debridement, if viable bone remains, the treatment should continue for approximately three months. In any case, the clinical evolution will determine the exact duration of the antibiotic treatment (38, 41).
TOPICAL TREATMENT
In recent years, new technologies and products have been developed to accelerate the healing of foot ulcers in patients with diabetes. Currently, there is a wide range of products and devices for topical treatment. The choice of the appropriate product depends on several factors, including the depth and extent of the ulcer, the presence of infection and/or necrotic tissue, and the degree of exudation (42). It is important to consider that the disease itself can slow down the healing process due to the presence of vasculopathy, neuropathy, humoral immunodeficiency factors, smoking, among others (3).
In ulcers located on the plantar surface of the foot, where the stratum corneum is thicker, the use of adhesive dressings is not recommended to avoid maceration. If the degree of exudation is significant or a graft is required, negative pressure therapy may be an effective therapeutic option (43). Illustration 7 provides guidance on topical treatment based on the grade of the ulcer.
Ilustración 7. Orientación sobre el tratamiento tópico de úlceras según el grado.
OFFLOADING SYSTEMS
Offloading is essential for the prevention or treatment of pressure areas or active ulcers. Various materials and systems are available for this purpose, requiring knowledge in biomechanics and skills for proper fabrication and application. The general objective of offloading, whether provisional (adhesive felt, plastic cast, functional orthosis) or definitive (plantar support, silicone orthosis, special footwear), is to evenly distribute the forces and pressures acting on the foot, protecting healthy areas and isolating ulcerated or susceptible areas (3).
Structural alterations of the foot, along with high plantar pressure, are major factors influencing the formation of plantar ulcers in diabetic patients (44). For the fabrication of provisional offloading systems, adhesive felt and polyurethane bandages are preferably used. The functional orthosis (Walker) can be used in combination with adhesive felt for offloading or to control edema and prevent deformities in patients with Charcot neuroarthropathy in phase 0-1 or suspected (45).
The use of plantar supports is indicated when the ulcer is healed or as a prevention for areas of high pressure that, if not corrected, could become ulcers, as well as to prevent recurrences (3, 46). Illustration 8 presents a detailed scheme of the different offloading systems used in the management of diabetic foot ulcers. These systems are crucial for reducing pressure on the affected areas and promoting healing.
Ilustración 8. Sistema de descargas de úlceras en pie diabético.
SURGICAL TREATMENT
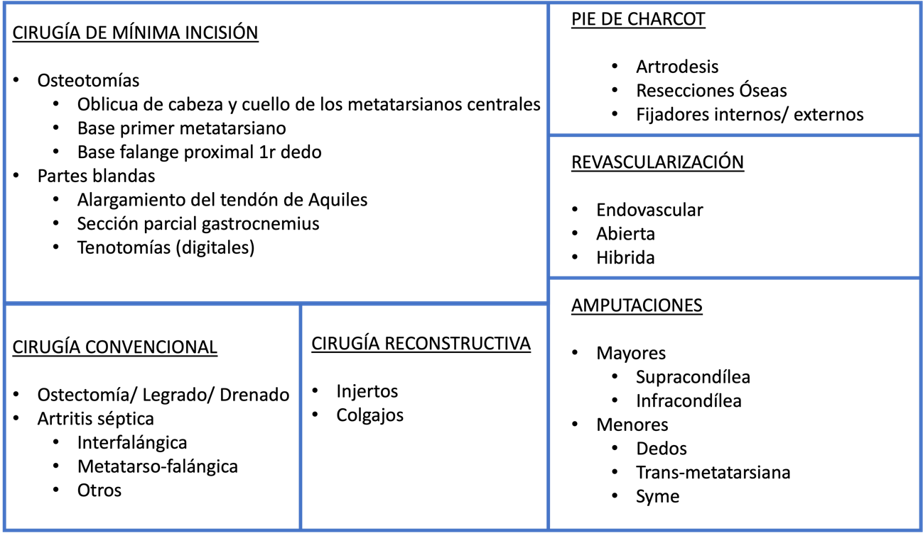
Surgery is often necessary in diabetic patients to address a variety of issues such as ulcers, infections, and severe deformities. Various surgical techniques are used to correct deformities, eliminate areas of high pressure, improve foot support, heal or prevent ulcers, revascularize the limb, or perform some type of amputation (47) (Illustration 9).
Ilustración 9. Técnicas quirúrgicas para el tratamiento del pie diabético.
The fundamental principle of surgical interventions in diabetic foot is osteotomies. These interventions allow for the correction of bone deformities that can contribute to ulcer formation. Surgery in these cases involves cutting and repositioning bones to relieve pressure and improve load distribution on the foot (48). Osteotomies are especially indicated in neuropathic ulcers without underlying osteomyelitis that do not respond to conventional treatment.
Among the most common osteotomies are: the base osteotomy of the first metatarsal (49), indicated in cases of hyperpressure on the head of the first metatarsal caused by cavus feet, adducted feet, posterior leg compartment muscle shortening, or biomechanical alterations; the base osteotomy of the proximal phalanx of the first toe (3), indicated for treating ulcers located on the plantar area of the first toe's interphalangeal joint; and the distal oblique osteotomy of the lesser metatarsals (second to fifth) (50), indicated for ulcers located in the plantar metatarsal area without the presence of osteomyelitis. The decision on the number and type of osteotomies is based on clinical and radiological criteria, considering the morphology of the metatarsal formula and the ulcer location, with the Leventen formula recommended to guide these interventions (3).
SURGICAL TECHNIQUES
When an infected foot is found, the most common surgical techniques initially include surgical debridement, which consists of removing dead or infected tissue around an ulcer. This procedure not only cleans the wound and promotes healing but also allows for sample collection for study and microbiological culture. Debridement may need to be repeated as necessary (3, 47, 51).
Once debridement is performed, various surgical techniques can be carried out depending on the specific problem. Partial or complete exostectomies allow for skin closure and prevent ulcer recurrence (3, 52). In cases of joint instability or major deformities, arthrodesis, which involves the fusion of a joint, is performed. By fusing a joint, its natural mobility is eliminated, thereby reducing the risk of new ulcer formation. Arthrodesis can be performed using internal fixation with plates and screws or external fixation (53), known as osteotaxis.
Due to the biological complexity of diabetic patients, arthrodesis is not always effective and may result in fibrous arthroplasty. Although not the ideal scenario, fibrous arthroplasty provides relative stability compatible with ambulation and a plantigrade foot (54). To avoid this scenario, current trends favor performing arthrodesis and osteosynthesis with "superconstruct" techniques, which use a greater amount of osteosynthesis material and screws to ensure more robust and durable fixation (55).
REVASCULARIZATION TECHNIQUES AND INDICATIONS
In situations where the results of other interventions are unsatisfactory and the evolution is unfavorable, compromising the limb or the patient's life (56), amputations are resorted to. In severe cases, where ulcers or infections are extensive and unresponsive to other treatments, amputation may be the only option to prevent infection spread and save the patient's life. Additionally, in cases where the arterial system is obstructed, revascularization of the diabetic foot may be necessary to restore blood flow. This may include techniques such as angioplasty, stent placement, or vascular bypass (3).
RECONSTRUCTION TECHNIQUES: PLASTIC SURGERY
Regarding plastic surgery, various techniques such as grafts, flaps, and artificial dermis are useful for ulcer coverage in patients with diabetic foot. Reconstruction of the distal lower extremity is a surgical challenge, especially in those cases with a high risk of complications such as diabetes or advanced vasculopathy. Several types of local and regional flaps have been described, such as the sural flap or the extensor digitorum flap. However, the main problem lies in the lack of reliability of their vascularization, especially in patients with underlying vasculopathy or loss of tissue quality due to chronic pathologies or the high thickness of the flap, creating contour defects (57).
FINAL RECOMMENDATIONS FOR THE DIAGNOSIS OF DIABETIC FOOT
• The diagnosis of diabetic foot infection should be primarily based on clinical criteria.
• A visible bone with a positive bone test is highly suggestive of osteitis/osteomyelitis.
• It is crucial to clean and debride before culturing.
• Biopsy is the most cost-effective procedure from a microbiological standpoint.
• For outpatient treatment, use antibiotics with good oral bioavailability and good compartmental distribution.
• An antibiotic window is recommended in case of poor evolution or recurrence (do not treat while selecting microorganisms).
• The microbiological documentation of diabetic foot infections is very helpful for antibiotic prescription, always accompanied by clinical information (especially appearance and evolution).
• The Microbiology laboratory should know, inform, and detect local bacterial resistance patterns.
• Gram staining as a rapid test provides valuable information. The quantification of cultures has not shown added value.
FINAL RECOMMENDATIONS FOR THE TREATMENT OF DIABETIC FOOT
• The treatment of diabetic foot infections should include empirical antibiotics covering Staphylococcus aureus (SSA, MRSA, CA-MRSA) and Streptococcus spp.
• It is essential to clean and debride the wound before taking samples for microbiological culture.
• Use antibiotics with good oral bioavailability and adequate compartmental distribution for outpatient treatment.
• In case of poor evolution or recurrence, an antibiotic window is recommended instead of continuously adjusting the treatment.
• Select products for topical treatment according to the ulcer's depth, extent, presence of infection or necrotic tissue, and exudate level.
• Implement offloading systems, such as adhesive felt, polyurethane bandages, and functional orthoses, to reduce pressure on the affected areas.
• Use plantar supports to prevent ulcer recurrence once healed and correct areas of hyperpressure.
• Perform surgical debridement to remove dead or infected tissue and promote healing, allowing sample collection for microbiological culture.
• Consider performing partial or complete exostectomies to allow skin closure and prevent recurrence.
• Employ arthrodesis to fuse joints in cases of instability or severe deformities, using internal or external fixation as needed.
CONCLUSIONS
Every patient diagnosed with diabetic foot should receive treatment through a multidisciplinary team (7-9, 58). It is essential to coordinate the different specialties with a common goal and establish a link between all specialists. The "System for the Evaluation and Treatment of Diabetic Foot" brings together in one document all the necessary variables for the proper management of these patients. This system can be used in any work setting, from primary care and diabetic foot units to hospitals and emergency services. Its implementation simplifies multidisciplinary management, facilitates collaboration among professionals, and significantly contributes to reducing the number of amputations. The results obtained over more than 20 years support its effectiveness and usefulness in clinical practice.
Authorship contributions:
Conception and design, critical revision of the manuscript, approval of the final version:
Luis Mas, Melanie Castro-Mallo. Critical revision of the manuscript, approval of the final
version: Rossana Ruiz, Katia Roque.
Financing:
Self-funded.
Declaration of conflict of interest:
The authors declare no conflict of interest.
Recevied:
January 5, 2024
Approved:
April 29, 2024
Correspondence author:
Jordi Viadé Julià
Address:
C/ Lacy 184. 08202. Sabadell. Barcelona.
Phone:
+34 630403613
E-mail:
jviadej@gmail.com
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES