ORIGINAL ARTICLE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2024 - Universidad Ricardo Palma10.25176/RFMH.v24i3.6538
EFFECT OF AVERRHOA CARAMBOLA L. ON THE SKIN IN AN ANIMAL MODEL OF HYPERTROPHIC SCARRING
EFECTO DE LA AVERRHOA CARAMBOLA L. EN LA PIEL EN UN MODELO ANIMAL DE CICATRIZACIÓN HIPERTRÓFICA
Alberto Córdova-Aguilar
 1,a,d,f
1,a,d,f
Elydia Mujica-Albán
 1,b,d,h
1,b,d,h
José Manuel Ortiz-Sánchez
 1,a,d,g
1,a,d,g
Silvia Suárez-Cunza
 2,c,j
2,c,j
Rodrigo Iglesias-Bustamante
 3,i
3,i
Daniel José Blanco-Victorio
 4,e,k
4,e,k
1 Faculty of Medicine, Universidad Nacional Mayor de San Marcos, Lima-Peru.
2 Faculty of Pharmacy and Biochemistry, Universidad Nacional Mayor de San Marcos, Lima-Peru.
3 Faculty of Veterinary Medicine, Universidad Nacional Mayor de San Marcos, Lima-Peru.
4 Faculty of Science and Engineering, Universidad Peruana Cayetano Heredia, Lima-Peru.
a Doctor of Medicine.
b Doctor of Science with a specialization in Physiology.
c Doctor of Pharmacy and Biochemistry.
d Master in Physiology.
e Master in Health Management and Specialist in Research.
f Medical Surgeon specializing in Plastic Surgery.
g Medical Surgeon specializing in Anatomical Pathology and Clinical Pathology.
h Biologist.
i Veterinarian.
j Pharmaceutical Chemist.
k Dental Surgeon.
ABSTRACT
Introduction: Hypertrophic scarring in the skin represents a serious global public health
problem, as it physically and emotionally affects people who suffer from it. This makes it necessary to
investigate new alternatives for its prevention and treatment. The objective of the study was to compare
the effect of Averrhoa Carambola L. (star fruit) on the skin under an animal model of hypertrophic
healing.
Methods: Experimental study; 10 male New Zealand breed rabbits were used, between 3 and 4 months
old, with an average weight of 3 to 3.5Kg. The formation of hypertrophic scars in the ears of rabbits was
induced under the model described by Morris et al. One of the groups received 1 mL of a 10% aqueous
solution of the lyophilized star fruit, while the other received 1 mL of triamcinolone acetonide; in both
groups the application was intralesional and weekly in a month. After the treatment, under sedation,
only the hypertrophic scars were extracted using a biopsy punch and the tissues were preserved in 10%
formalin for subsequent pathological examination.
Results: The group that received star fruit solution significantly improved the dermis and
epidermis in the hypertrophic scars on the ears of rabbits that received treatment. When compared with
the group that received triamcinolone acetonide, there were no statistically significant differences.
Conclusion: The lyophilized aqueous extract of star fruit at 10% demonstrated a similar effect
compare to triamcinolone acetonide (treatment of choice) in reducing the fibrosis of hypertrophic scars
in the ears of rabbits.
Keywords: Averrhoa Carambola L., triamcinolone, hypertrophic scar, animal models. (MeSH/NLM)
RESUMEN
Introducción: La cicatrización hipertrófica en la piel representa un grave problema de salud
pública mundial, pues afecta física y emocionalmente a las personas que la padecen. Esto hace necesario
investigar nuevas alternativas para su prevención y tratamiento. El objetivo del estudio fue comparar el
efecto de la Averrhoa Carambola L. (carambola) en la piel bajo un modelo animal de cicatrización
hipertrófica.
Métodos: Estudio experimental; se utilizó 10 conejos machos de raza Nueva Zelanda, entre 3 y 4
meses de edad, con un peso promedio de 3 a 3.5Kg. Se indujo la formación de cicatrices hipertróficas en
las orejas de los conejos bajo el modelo descrito por Morris y col. A un grupo de heridas se administró
1mL de solución acuosa del liofilizado del fruto de la carambola al 10%, mientras que al otro grupo de
heridas se administró 1mL del acetónido de triamcinolona; en ambos grupos la aplicación fue intralesional,
semanal y por un mes consecutivo. Finalizado el tratamiento, bajo sedación se extrajeron solo las
cicatrices hipertróficas utilizando un punzón de biopsia y los tejidos fueron conservados en formol al
10% para el examen anatomopatológico posterior.
Resultados: El grupo que recibió la solución de carambola al 10% mejoró significativamente la
dermis y la epidermis en las cicatrices hipertróficas de las orejas de conejos que recibieron
tratamiento. Al compararla con el grupo que recibió el acetónido de triamcinolona, no hubo diferencias
estadísticamente significativas.
Conclusión: El extracto acuoso liofilizado de carambola al 10% demostró un efecto similar al
acetónido de triamcinolona (tratamiento de elección) en la reducción de la fibrosis de las cicatrices
hipertróficas en las orejas de los conejos.
Palabras clave: Averrhoa Carambola L.; triamcinolona; cicatriz hipertrófica; modelos animales.
(Fuente: DeCS/BIREME)
INTRODUCTION
Hypertrophic scarring in the skin results from excessive collagen deposition over a scar and can cause severe functional and aesthetic issues, such as movement limitation, pain, pruritus, and chronic inflammation of the affected area. This type of scarring is common in humans, particularly in postoperative and deep burn patients. In industrialized countries, around 100 million people annually develop excessive skin scars or related problems. This issue is so significant that the anti-scar drug market exceeds 12 billion US dollars annually (1, 2).
Physiological skin healing is a tissue repair process involving three phases (inflammation, proliferation, and remodeling) and lasts for a year. It appears that if the inflammatory phase is prolonged or excessive, hypertrophic scarring occurs, characterized histologically by increased fibroblasts, extracellular matrix, and neoangiogenesis (3, 4, 5).
Currently, the lack of understanding of the pathogenesis leads to the use of various nonspecific therapies, such as triamcinolone acetonide. This synthetic corticosteroid, applied intralesionally, has become the first choice for treating hypertrophic scars, although it can produce rebound effects, hypopigmentation, and even atrophy of the underlying skin and fat (1, 6, 7).
Although hypertrophic scarring in the skin is an almost exclusive problem for humans, delayed epithelialization in wounds on the ventral skin of rabbit ears produces hypertrophic scars similar to those in humans (8, 9). This allows the use of this animal model for studying hypertrophic scarring.
Peru has a megadiversity of medicinal plants that can be used as potential phytopharmaceuticals. One of these plants is Averrhoa carambola L., especially cultivated in the Amazon, and its fruit is popular as a moisturizer. In traditional medicine, it is primarily used as a hypoglycemic, antihypertensive, antitussive, and antiemetic (10).
Various extracts of Averrhoa carambola L. at different concentrations and administration routes have demonstrated anti-inflammatory and antioxidant activities, producing nephroprotective, cardioprotective, and hepatoprotective effects in experimental animals, although the active principle of this biological activity is unknown (11-15).
Currently, there are no studies on the anti-inflammatory effect of Averrhoa carambola L. fruit on hypertrophic scars in rabbit ears. Therefore, the objective of this research was to compare the effects of Averrhoa carambola L. with triamcinolone acetonide on induced hypertrophic scars in rabbit ears.
METHODSType, design, and study area
The research was basic, with a preclinical experimental design in the area of experimental physiology.
Population and sample
The sample consisted of 40 hypertrophic scars on the ears of New Zealand rabbits. Ten male rabbits, aged
between 3 and 4 months, with an average weight of 3 to 3.5 kg, were used. They were obtained from the
Instituto Nacional de Salud (Chorrillos, Lima, Peru).
Experimental design and procedures
Due to the restrictions established during the COVID-19 pandemic, a temporary bioterium was set up in
Chorrillos, considering space, ventilation, and temperature. The rabbits were kept in individual cages
at 24°C and 60% humidity, with 12-hour light/dark cycles, receiving free access to water and food. The
food was acquired from the Universidad Agraria La Molina, and the care of the animals was overseen by a
veterinarian.
The lyophilized extract of Averrhoa carambola L. was obtained from NutriCargo® and reconstituted to 10% in the laboratory of the Instituto de Investigación de Bioquímica y Nutrición “Alberto Guzmán Barrón” (Faculty of Medicine – UNMSM).
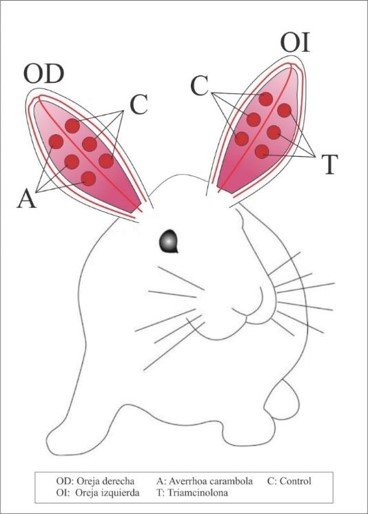
The hypertrophic scarring model in rabbit ears was conducted as described by Morris et al. (16). Forty hypertrophic scars were induced on the ventral skin of the rabbits' ears using 6mm biopsy punches, left to heal for 8 weeks. Once hypertrophic scars formed, 20 of them (2 wounds per ear) were randomly selected to receive 1mg of treatment A or B intralesionally for four consecutive weeks, as per the scheme in Figure 1. Triamcinolone acetonide (Triamcicort®) was administered to the hypertrophic scars on the left ears, and lyophilized aqueous extract of Averrhoa carambola L. (NutriCargo®) was administered to the scars on the right ears. At 90 days of the trial, under sedation, only the hypertrophic scars were excised using a 7mm biopsy punch, and the tissues were preserved in 10% formalin for subsequent histopathological examination.
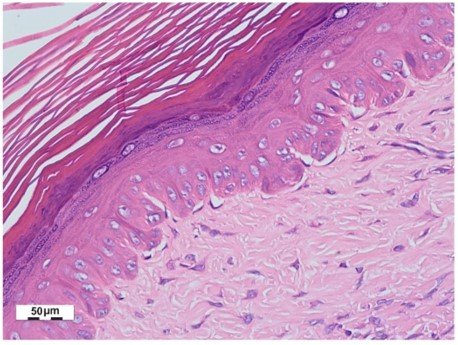
The reduction in thickness in the photomicrographs of the papillary and reticular dermis of the hypertrophic scars was evaluated using an Olympus BX53F® optical microscope with hematoxylin-eosin staining. The distance between the crest and the papilla was measured at 40X in the papillary dermis, and the total thickness was measured at 10X in the reticular dermis. The dermal measurements in the photomicrographs were performed on a scale (in µm) using CorelDRAW 2020® software.
Figure 1: Treatment applied to rabbit ears.
Figure 2: Measurement method of the papillary dermis (left) and reticular dermis (right) in CorelDRAW 2020® program
Statistical analysis
The data obtained were recorded on a data sheet and imported into a Microsoft Excel 2021® spreadsheet for subsequent tabulation and analysis with the statistical program Stata® v17.0. The data were processed using descriptive and inferential statistics. Numerical variables were analyzed with measures of central tendency and dispersion. Medians of both groups were compared using the non-parametric Wilcoxon test. The final report was prepared using Microsoft Word 2021®.
Ethical aspectsThis research was approved by the Graduate Unit of the Faculty of Medicine of the Universidad Nacional Mayor de San Marcos (Resolution No. 000374-2021-UPG-VDIP-FM/UNMSM). The study adhered to international ethical principles for research with laboratory animals and was based on the 3Rs: refinement, reduction, and replacement. Furthermore, it followed the guidelines of the Council for International Organizations of Medical Sciences, as outlined in the guide for the care and use of laboratory animals (17).
RESULTSThe mean hypertrophic scar (papillary dermis: crest-to-papilla distance) in the group that received Averrhoa carambola L. was 32.3 µm (±17.21), and in the group that received triamcinolone acetonide, it was 22.05 µm (±20.15). Evaluation with the Wilcoxon test showed no statistically significant differences (Table 1).
|
Group |
Mean |
SD |
Median |
IQR |
Min |
Max |
n |
p |
|---|---|---|---|---|---|---|---|---|
| Star fruit | 32.3 | 17.21 | 31.50 | 21 | 8 | 57 | 10 | 0.414* |
| Triamcinolone | 22.05 | 20.15 | 20.75 | 38 | 0 | 60 | 10 |
*Wilcoxon test, Z=-0.868, p>0.05 not significant
The mean hypertrophic scar (reticular dermis) in the group that received Averrhoa carambola L. was 291.60 µm (±61.56), and in the group that received triamcinolone acetonide, it was 292.40 µm (±78.83). Evaluation with the Wilcoxon test showed no statistically significant differences (Table 2).
|
Group |
Mean |
SD |
Median |
IQR |
Min |
Max |
n |
p |
|---|---|---|---|---|---|---|---|---|
| Star fruit | 291.60 | 61.56 | 262.5 | 99 | 228 | 395 | 10 | 0.941* |
| Triamcinolone | 292.40 | 78.83 | 324 | 114 | 168 | 400 | 10 |
*Wilcoxon test, Z= -0.102, p>0.05 not significant
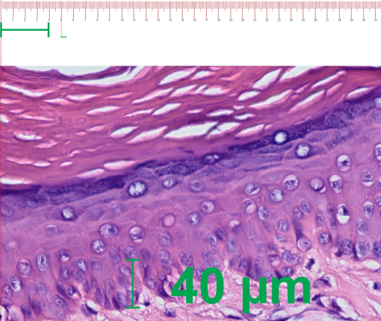
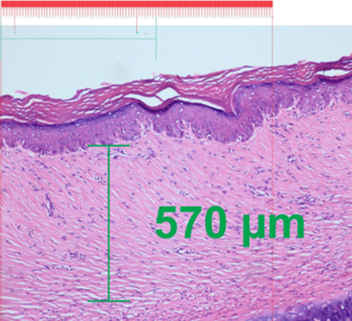
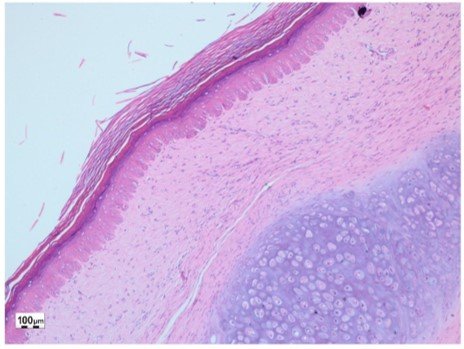
The effect of the 10% lyophilized aqueous extract of star fruit on hypertrophic scars after 1 month of weekly treatment is shown in Figures 3 and 4. Figure 3 shows an increase in the papillary dermis (greater crest-to-papilla distance), and Figure 4 shows a decrease in the thickness of the reticular dermis (shorter distance from papillary dermis to hypodermis).
Figure 3: Post-treatment scar with Averrhoa Carambola L., hematoxylin-eosin (40X)
Figure 4: Post-treatment scar with Averrhoa carambola L., hematoxylin-eosin (10X),
DISCUSSION
Hypertrophic scarring in the skin affects all wounds that reach the reticular dermis. Despite the multifactorial etiology of hypertrophic scars, various factors point to chronic inflammation caused by free radicals as the main cause. In this sense, the study of plant-derived compounds with anti-inflammatory and antioxidant potential, such as Averrhoa carambola L. (star fruit), could offer new treatments (10-14,18-22).
The data obtained demonstrate that both Averrhoa carambola L. (star fruit) and triamcinolone acetonide improved healing in a hypertrophic scar model in rabbit ears. This finding is similar to other studies that showed improvement in hypertrophic scarring with 10% simvastatin cream, kiwi, apremilast, asiaticoside, usnic acid, botulinum toxin type A, and dimethyl sulfoxide (23-29).
Comparing the medians obtained from the crest-to-papilla distance in the papillary dermis, no statistically significant differences were found between the group that received Averrhoa carambola L. and the group that received triamcinolone acetonide. Similarly, comparing the medians obtained from the total thickness in the reticular dermis of both groups, no statistically significant differences were found. This result is relevant as it demonstrates that Averrhoa carambola L. had positive effects in the treatment of hypertrophic scars in rabbit ears.
The papillary dermis, being the most superficial layer of the dermis, is in direct contact with the epidermis and contains a large number of blood vessels, nerves, and dermal stem cells that can differentiate into various types of skin cells, including fibroblasts that produce collagen and other components of the extracellular matrix. This makes it an important region for skin repair and regeneration. Similarly, the reticular dermis, being the deepest layer of the dermis, provides structure and strength to the skin, although its regenerative capacity is limited compared to the papillary dermis (1-2).
Analyzing the results of both treatments specifically, it was observed that Averrhoa carambola L. (star fruit) had a better effect on the papillary dermis compared to triamcinolone acetonide, which had a better effect on the reticular dermis. This significant effect of Averrhoa carambola L. (star fruit) on the papillary dermis seems to be due to the high antioxidant activity of the plant, given its high content of vitamin C, polyphenols, and flavonoids. These positive results were obtained with an aqueous extract from a commercial lyophilized product of Averrhoa carambola L. (NutriCargo®), which reinforces the idea that lyophilized materials maintain their organoleptic properties.
Conversely, triamcinolone acetonide had a better effect on the reticular dermis. This is likely due to its strong antiproliferative activity, resulting from the inhibition of phospholipase A2 and the subsequent inhibition of the inflammatory cascade. It is likely to reduce the expression of genes encoding pro-inflammatory cytokines and adhesion molecules, thereby decreasing the infiltration of inflammatory cells at the inflammation site. The reticular dermis has thicker and more rigid collagen fibers. Various studies have demonstrated that triamcinolone acetonide reduces fibroblast activity, and thus the synthesis of collagen and other components of the extracellular matrix, reducing the amount and stiffness of collagen fibers in the scar (7,8,30). It has also been shown that triamcinolone acetonide inhibits the formation of new blood vessels (neoangiogenesis) in hypertrophic scars, reducing inflammation and redness of the scar.
Although previous studies indicate that Averrhoa carambola L. (star fruit) has anti-inflammatory and antioxidant activity, its greatest effect appears to be the latter.
The main strength of the study lies in being the first investigation in the country, as far as reviewed, to use Averrhoa carambola L. (star fruit) for the treatment of hypertrophic scars in rabbit ears. Additionally, the main limitation of the study was the COVID-19 pandemic. It was necessary to implement a temporary bioterium for the care of the experimental animals and work with the lyophilized product.
Finally, while the results cannot be directly extrapolated to humans, it opens a promising path for further investigation into the benefits of this potential phytopharmaceutical and its beneficial effects on pathological scarring.
Authorship contribution: The authors participated in the conception of the idea, project
design, data collection and interpretation, result analysis, and preparation of the manuscript
for this research work.
Funding: Self-funded.
Conflict of interest statement: The authors declare no conflicts of interest.
Received: April 2nd, 2024
Approved: June 28th, 2024
Corresponding author: Alberto Córdova-Aguilar
Address: Jr. Paseo del Prado 133 – Urb. Las Lomas, La Molina. Lima, Peru.
Phone number: (511) 999 779 789
E-mail: acordovaa@unmsm.edu.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES