ORIGINAL ARTICLE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2024 - Universidad Ricardo Palma10.25176/RFMH.v24i2.6558
PRONOSTIC CAPACITY OF D-DIMER IN PREDICTING MORTALITY IN PATIENTS DIAGNOSED WITH COVID-19
CAPACIDAD PRONÓSTICA DEL DIMERO D EN LA PREDICCIÓN DE MORTALIDAD EN PACIENTES CON EL DIAGNÓSTICO DE COVID-19
Joseph Alburqueque-Melgarejo
 1,a,
Israel Armando Guerra Cuyutupac
1,a,
Israel Armando Guerra Cuyutupac
 1,b,
Juan Carlos Ezequiel Roque-Quezada
1,b,
Juan Carlos Ezequiel Roque-Quezada
 1,c,
Horus Michael Virú Flores
1,c,
Horus Michael Virú Flores
 3
Martha Eugenia Aguirre Coronado
3
Martha Eugenia Aguirre Coronado
 2
Luis Enrique Nieves Cordova
2
Luis Enrique Nieves Cordova
 4
4
1 Universidad Cientifica del Sur. Lima, Perú.
2 Hospital de Emergencias José Casimiro Ulloa. Lima, Perú.
3 Facultad de Medicina Humana, Universidad Privada San Juan Bautista. Lima, Perú.
4 Centro Médico Naval. Lima, Perú
a Médico Cirujano
b b Doctor en Educación
c Magister en Medicina Humana
ABSTRACT
Introduction: SARS-CoV-2 infection can increase the risk of thrombosis. Studies associate D-dimer levels with COVID-19 mortality.
Objective: To determine the prognostic capacity of D-dimer in predicting COVID-19 mortality in patients hospitalized in the Intensive Care Unit.
Methods: A retrospective cohort study was conducted at the Naval Medical Center from January to July 2021. A total of 324 adult patients with RT-PCR confirmed COVID-19 were included. D-dimer levels were measured upon admission using coagulation turbidimetry (Sysmex CS-5100). Sociodemographic variables, comorbidities, and clinical data were analyzed. Statistical analysis was performed using SPSS version 26, employing Chi-square tests, Fisher's exact test, Mann-Whitney U test, ROC, and Cox regression.
Results: A cut-off point of 1.40 µg/mL for D-dimer values was determined, with a sensitivity of 80.9%, specificity of 86.4%, and an area under the curve (AUC) of 0.916 (95% CI: 0.884 – 0.947; p=0.016) for predicting COVID-19 mortality. Additionally, patients with D-dimer values greater than or equal to 1.40 µg/mL had an increased risk of death (adjusted HR = 6.545; 95% CI: 3.867 – 11.077; p<0.001), independent of age, diabetes mellitus, arterial hypertension, ischemic heart disease, cerebrovascular disease, atrial fibrillation, chronic obstructive pulmonary disease, asthma, cancer, and thrombocytopenia.
Conclusion: This study showed that admission D-dimer levels represent a reliable biomarker in evaluating the prognosis of COVID-19 patients.
Keywords: COVID-19; SARS-CoV-2; Mortality; Fibrin-Fibrinogen Degradation Products (Source: MeSH NLM).
Keywords: COVID-19; SARS-CoV-2; Mortality; Fibrin-Fibrinogen Degradation Products. (source: MeSH
NLM)
RESUMEN
Introducción: La infección por SARS-CoV-2 puede aumentar el riesgo de trombosis. Estudios asocian niveles de dímero D con mortalidad por COVID-19.
Objetivo: Determinar la capacidad pronostica del dimero D en la predicción de mortalidad por COVID-19 en pacientes hospitalizados en la Unidad de Cuidados Intensivos.
Métodos: Se realizó un estudio de cohorte retrospectiva en el Centro Médico Naval durante enero-julio de 2021. Se incluyeron 324 pacientes adultos con COVID-19 confirmada por RT-PCR. Se midieron niveles de dímero D al ingreso utilizando turbidimetría de coagulación (Sysmex CS-5100). Se analizaron variables sociodemográficas, comorbilidades y datos clínicos. El análisis estadístico se realizó con SPSS versión 26, empleando pruebas Chi cuadrado, exacta de Fisher, U de Mann Whitney, COR y regresión de Cox.
Resultados: Se determinó un punto de corte de 1,40 µg/mL para los valores de dimero D con una sensibilidad de 80,9%, una especificidad de 86,4% y área bajo la curva (AUC) de 0,916 (IC 95%: 0,884 – 0,947; p =0,016) para predecir mortalidad por COVID-19. Asimismo, se encontró que pacientes con valores de dimero D mayores o iguales a 1,40 µg/mL tenian un riesgo incrementado de fallecimiento en pacientes con COVID-19 (HRa = 6,545; IC 95%: 3,867 – 11,077; p<0,001), independientemente de las variables edad, diabetes mellitus, hipertensión arterial, cardiopatía isquémica, enfermedad cerebrovascular, fibrilación auricular, enfermedad pulmonar obstructiva crónica, asma, cáncer y trombocitopenia.
Conclusión: El presente estudio mostró que los niveles de dimero D al ingreso representan un biomarcador fiable en la evaluación del pronostico de pacientes con COVID-19.
Palabras clave: COVID-19; SARS-CoV-2; Mortalidad; Productos de Degradación de Fibrina-Fibrinógeno.
(fuente: DeCS-BIREME)
INTRODUCTION
The infection caused by the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) can affect multiple organ systems, including the respiratory, cardiac, gastrointestinal, hepatic, neurological, hematological, and renal systems (1 - 3). Several studies have reported the presence of multiple thrombotic complications, both arterial and venous, especially in patients with comorbidities such as hypertension, diabetes mellitus, obesity, and neoplasms. Among these thrombotic complications, disseminated intravascular coagulation, pulmonary venous thromboembolism, deep vein thrombosis, myocardial infarction, large vessel cerebral infarction, acute limb ischemia, catheter-associated thrombosis, and arterial and venous thromboembolism stand out (4 - 8).
These complications can be explained by the virus's ability to infect endothelial cells, produce endotheliitis, and cause endothelial dysfunction, establishing a subsequent prothrombotic state, explained by Virchow's triad. Additionally, SARS-CoV-2 can activate platelets and induce a proinflammatory phenotype that optimizes their interactions with the endothelium and circulating leukocytes, promoting the activation of the coagulation cascade, the complement system, and the formation of neutrophil extracellular traps (NETs). These alterations, along with the cytokine storm and the possibility of an antiphospholipid syndrome related to the viral infection, have been shown to play a role in the pathogenesis of the prothrombotic state induced by SARS-CoV-2 (1, 9, 10).
The presence of thromboembolic complications has been associated with high mortality in patients hospitalized in the Intensive Care Unit (ICU) for COVID-19 (11, 12). For this reason, the use of biomarkers to estimate the prognosis of these patients has been proposed. One of these biomarkers is D-dimer, a fibrin degradation product, which has shown prognostic utility in the evaluation of COVID-19 patients. Several studies around the world have shown the association between D-dimer levels at admission and the development of mortality from COVID-19 (13 - 16). Therefore, the objective of this study was to determine the prognostic capability of D-dimer in predicting mortality in patients hospitalized in the ICU of the Centro Médico Naval during the period from January to July 2021.
Methodology
Study design and area
This study employed an observational, analytical, and retrospective cohort design. The STROBE checklist for cohort studies was applied (17). The Centro Médico Naval is a level III healthcare facility in an urban setting that serves insured patients of the Peruvian Navy, both primary members and their families.
Population and Sample
The study population consisted of adult patients diagnosed with COVID-19, confirmed by reverse transcription polymerase chain reaction (RT-PCR), who were hospitalized in the ICU of the Centro Médico Naval during the period from January to July 2021. The selection criteria included adult patients admitted to the ICU with D-dimer values at admission and whose medical history contained the variables of interest for the study. Patients with incomplete medical records were excluded.
Sample size
The sample size was calculated using the Epidat statistical software version 4.2. The operational characteristics of sensitivity and specificity were 92.3% and 83.3%, respectively, and were extracted from the study conducted by Zhang et al. (14). A 95% confidence level and an absolute precision of 5% were established. As a result, a sample size of 324 patients was determined. These participants were divided into two groups according to their prognosis, with one group consisting of 110 deceased patients and the other group consisting of 214 non-deceased patients.
Sampling method
A simple random probabilistic sampling without replacement was performed using the Epidat epidemiological package version 4.2 to select 324 patients diagnosed with COVID-19 hospitalized in the ICU.
Variables and Instruments
Data on the study variables were collected from each patient's medical history within 24 hours of hospital admission.
Covariables
Sociodemographic and comorbidity variables included age, sex, hypertension, type 2 diabetes mellitus, hypothyroidism, ischemic heart disease, cerebrovascular disease, atrial fibrillation, tuberculosis, chronic obstructive pulmonary disease (COPD), asthma, and cancer, and were evaluated as dichotomous qualitative variables. Similarly, clinical variables such as fever, cough, headache, dyspnea, pharyngodynia, anosmia, diarrhea, and adult respiratory distress syndrome (ARDS) were evaluated as dichotomous qualitative variables. The variables D-dimer, platelet count, prothrombin time, partial thromboplastin time, and fibrinogen were evaluated as continuous quantitative variables.
Exposure variable
D-dimer levels were measured using a Sysmex CS-5100 coagulation analyzer, and the technique employed was clotting turbidimetry. The reference range for normal D-dimer levels was between 0 and 0.5 µg/mL. All patients received treatment with low-molecular-weight heparin in prophylactic doses and full doses as appropriate.
Outcome variable
The disease outcome was evaluated according to the occurrence of the event of interest for the study within 30 days of hospitalization and was defined as a dichotomous qualitative variable with categories corresponding to death or recovery. Patients who did not present the event during the follow-up period were classified as censored. Mortality was evaluated according to its definition by the Pan American Health Organization as death occurring as a result of a disease in a probable or confirmed case of COVID-19.
Statistical analysis
The collected data were entered into a database using SPSS version 26 for statistical analysis. The descriptive analysis included frequency tables for qualitative variables, where absolute and relative frequencies were reported. Since the quantitative variables did not present a normal distribution, the median and interquartile range (IQR) were reported. Inferential analysis included Chi-square and Fisher's exact tests for qualitative variables. For quantitative variables, the Mann-Whitney U test for unrelated samples was used. The optimal cut-off point and the receiver operating characteristic (ROC) curve with their respective operational characteristics for the D-dimer variable were calculated. Additionally, a Kaplan-Meier survival analysis was performed, from which the log-rank test was calculated. Subsequently, bivariate and multivariate analyses were conducted using the Cox proportional hazards model with their respective crude (HR) and adjusted hazard ratios (aHR), 95% confidence intervals (95% CI), and p-values.
Ethical Aspects
For the execution of this research project, approval was obtained from the Ethics Committee of the Centro Médico Naval Cirujano Mayor Santiago Távara. As it was a study involving patients, confidentiality and patient rights were respected in accordance with bioethical principles and the Declaration of Helsinki.
Results
Of the 324 patients included in the study, the median age was 55 years (IQR: 17), with ages ranging from 20 to 87 years. Deceased COVID-19 patients aged 60 years or older represented 17.3%. It was observed that male patients (29.3%) had a higher percentage of deaths compared to female patients (4.6%). Table 1 describes the sociodemographic, epidemiological, and clinical characteristics of the patients comprising the study sample.
| COVID-19 Patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Deceased | Survivors | p-value | ||||||
| n | % | n | % | ||||||
| Age | |||||||||
| < 60 years | 54 | 16,7% | 184 | 56,8% | 0,087 | ||||
| >= 60 years | 56 | 17,3% | 30 | 9,3% | |||||
| Sex | |||||||||
| Male | 95 | 29,3% | 168 | 51,9% | < 0,001 | ||||
| Female | 15 | 4,6% | 46 | 14,2% | |||||
| Hypertension | 18 | 5,6% | 10 | 3,1% | < 0,001 | ||||
| Type 2 Diabetes mellitus | 27 | 8,3% | 8 | 2,5% | < 0,001 | ||||
| Hypothyroidism | 6 | 1,9% | 5 | 1,5% | 0,194 | ||||
| Ischemic heart disease | 18 | 5,6% | 5 | 1,5% | < 0,001 | ||||
| Cerebrovascular disease | 7 | 2,2% | 4 | 1,2% | 0,049 | ||||
| Atrial fibrillation | 18 | 5,6% | 3 | 0,9% | < 0,001 | ||||
| Tuberculosis | 5 | 1,5% | 6 | 1,9% | 0,519 | ||||
| COPD | 24 | 7,4% | 13 | 4% | < 0,001 | ||||
| Asthma | 26 | 8% | 15 | 4,6% | < 0,001 | ||||
| Cancer | 20 | 6,2% | 8 | 2,5% | < 0,001 | ||||
| Fever | 96 | 29,6% | 135 | 41,7% | 0,001 | ||||
| Cough | 98 | 30,2% | 153 | 47,2% | < 0,001 | ||||
| Headache | 89 | 27,5% | 135 | 41,7% | 0,005 | ||||
| Dyspnea | 93 | 29,1% | 159 | 49,7% | 0,067 | ||||
| Pharyngodynia | 14 | 4,3% | 53 | 17,3% | < 0,001 | ||||
| Anosmia | 16 | 4,9% | 32 | 9,9% | 0,099 | ||||
| Diarrhea | 14 | 4,3% | 35 | 10,8% | 0,418 | ||||
| ARDS | 110 | 34% | 204 | 63% | < 0,001 | ||||
Regarding laboratory variables, statistically significant differences (p-value < 0.005) were found between the deceased and surviving patient groups for the variables D-dimer, platelet count, prothrombin time, partial thromboplastin time, and fibrinogen (Table 2).
| COVID-19 Patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Deceased (n = 110) | Survivors (n = 214) | p value* | ||||||
| Median | IQR | Median | IQR | ||||||
| D-dimer (µg/mL) | 1,73 | 0,52 | 0,98 | 0,75 | < 0,001 | ||||
| Platelet count (cells/mm³) | 135 | 74,25 | 219 | 75 | < 0,001 | ||||
| Prothrombin time (s) | 17 | 5,25 | 13 | 3 | < 0,001 | ||||
| Partial thromboplastin time (s) | 33 | 12 | 29 | 9 | < 0,001 | ||||
| Fibrinogen (mg/dL) | 682,0 | 282,85 | 550,5 | 211,5 | < 0,001 | ||||
A ROC curve was created for the D-dimer variable to discriminate between death and recovery in COVID-19 patients. From this, a cut-off point of 1.40 µg/mL was obtained with a sensitivity of 80.9%, a specificity of 86.4%, and a Youden index of 0.67. Additionally, an area under the curve (AUC) of 0.916 (95% CI: 0.884–0.947; p-value = 0.016) was obtained to predict mortality (Figure 1).
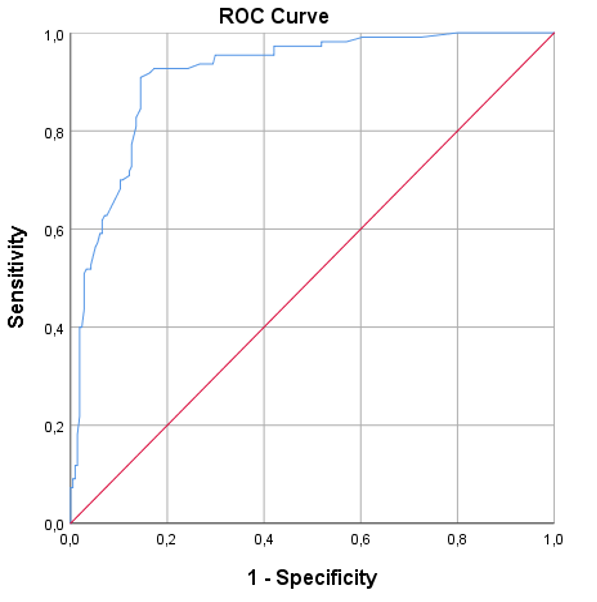
It was found that 89 patients (27.5%) with D-dimer levels greater than or equal to 1.40 µg/mL died, compared to 29 (9%) who did not die. The Kaplan-Meier survival curves for D-dimer levels, based on the cut-off point of 1.40 µg/mL to estimate 30-day survival, showed statistically significant differences between the high D-dimer and low D-dimer groups, according to the log-rank (Mantel-Cox) test (chi-square = 169.868; p-value < 0.001) (Figure 2).
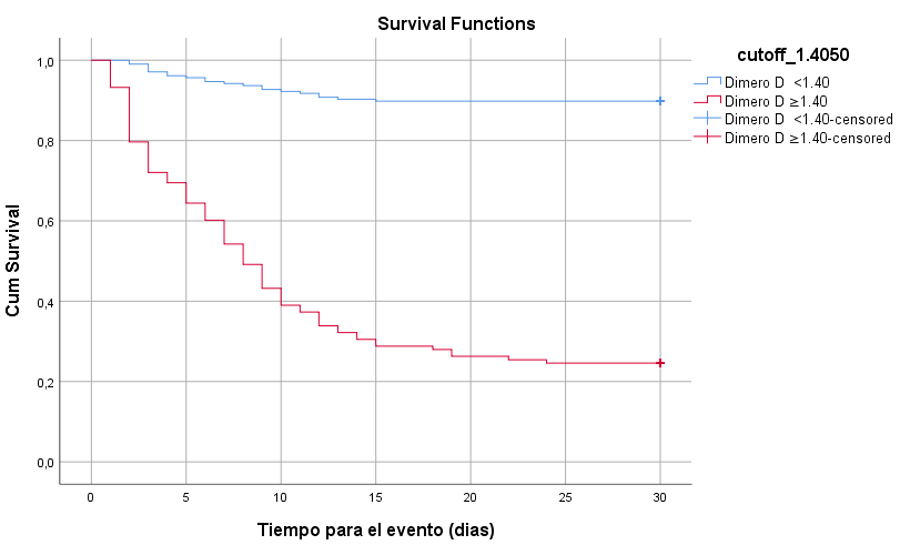
To analyze if D-dimer levels greater than 1.40 µg/mL had predictive utility in 30-day survival, a univariate and multivariate Cox proportional regression model was conducted, adjusting for potential confounding variables. It was found that D-dimer levels greater than 1.40 µg/mL increased the risk of death in COVID-19 patients (aHR = 6.55; 95% CI: 3.87–11.08; p-value < 0.001), independent of variables such as age, diabetes mellitus, hypertension, ischemic heart disease, cerebrovascular disease, atrial fibrillation, COPD, asthma, cancer, and thrombocytopenia (Table 3).
| Variable | Crude HR | p value | Adjusted HR | p value | ||||
|---|---|---|---|---|---|---|---|---|
| HR | CI 95% Lower | CI 95% Upper | HR | CI 95% Lower | CI 95% Upper | |||
| D-dimer > 1.4 | 7,86 | 5,28 | 11,69 | < 0,001 | 6,55 | 3,87 | 11,08 | < 0,001 |
| Age > 60 years | 3,79 | 2,60 | 5,52 | < 0,001 | 1,32 | 0,83 | 2,09 | 0,241 |
| Male sex | 1,62 | 0,94 | 2,79 | 0,083 | ||||
| Diabetes Mellitus | 3,86 | 2,49 | 5,99 | < 0,001 | 1,53 | 0,84 | 2,78 | 0,165 |
| Hypertension | 2,59 | 1,56 | 4,29 | < 0,001 | 1,38 | 0,73 | 2,61 | 0,322 |
| Hypothyroidism | 1,61 | 0,71 | 3,68 | 0,254 | ||||
| Ischemic heart disease | 3,35 | 2,02 | 5,57 | < 0,001 | 1,87 | 1,11 | 3,16 | 0,020 |
| Cerebrovascular disease | 2,43 | 1,13 | 5,22 | 0,024 | 2,04 | 0,89 | 4,67 | 0,091 |
| Atrial fibrillation | 4,11 | 2,47 | 6,85 | < 0,001 | 1,90 | 1,04 | 3,45 | 0,036 |
| Tuberculosis | 1,62 | 0,34 | 3,10 | 0,690 | ||||
| COPD | 2,37 | 1,51 | 3,71 | < 0,001 | 2,34 | 1,39 | 3,92 | 0,002 |
| Asthma | 2,76 | 1,71 | 4,45 | < 0,001 | 2,40 | 1,38 | 4,17 | 0,002 |
| Cancer | 3,47 | 2,02 | 5,95 | < 0,001 | 3,05 | 1,74 | 5,35 | < 0,001 |
| Platelets < 150 (Thrombocytopenia) | 6,58 | 4,35 | 9,97 | < 0,001 | 3,41 | 2,23 | 5,52 | < 0,001 |
DISCUSSION
In this study, it was found that D-dimer levels at admission greater than or equal to 1.40 µg/mL increased the probability of mortality from COVID-19 independently of the other covariates evaluated (aHR = 6.55; 95% CI: 3.87 – 11.08; p-value < 0.001). Several studies worldwide have demonstrated the association between D-dimer levels at admission and disease severity and outcome in COVID-19 patients (18 - 20).
A study conducted in Nepal by Poudel A. et al., which included a total of 182 patients, showed that D-dimer levels at admission above 1.5 µg/mL were significantly associated with the mortality variable (aHR = 5.86; 95% CI: 2.75 – 12.49; p-value < 0.001) independently of the age variable (21).
Zhang L. et al., conducted a study in China that included 343 subjects, finding that D-dimer levels at admission above 2 µg/mL predicted in-hospital mortality in patients hospitalized for COVID-19 (aHR = 22.40; 95% CI: 2.86 – 175.70; p-value = 0.003) (14).
Similarly, the study by Soni M. et al., conducted in a population of 483 patients, showed that D-dimer levels above 2.01 µg/mL were significant predictors of mortality (aHR = 3.17; 95% CI: 2.01 – 4.98; p-value < 0.01) (22). These results underline the importance of D-dimer as a prognostic marker in COVID-19 patients. The consistency of findings across different studies reinforces the validity of the results obtained in this investigation. Monitoring D-dimer levels could be crucial in the clinical management of these patients.
A retrospective cohort study conducted by Tang et al., in a population of 449 patients with severe COVID-19, reported that D-dimer levels were associated with 28-day in-hospital mortality (OR = 1.06; 95% CI: 1.03 – 1.09; p-value < 0.001) (23). A systematic review by Sakka M. et al., examining a total of six original studies in hospitalized COVID-19 patients, demonstrated that D-dimer levels were significantly associated with an increased risk of mortality (SMD = 3.59; 95% CI: 2.79 – 4.40; p-value < 0.00001) (24). These findings are consistent with those found in the present study. The results of these studies support the clinical relevance of D-dimer as a risk indicator in hospital practice. Additionally, they underscore the need to incorporate D-dimer measurement into evaluation protocols for COVID-19 patients. Systematic evaluation of this marker could significantly improve treatment and management strategies.
In contrast to the findings of the present study, Cidade J. et al., conducted a single-center retrospective cohort study in a sample of 118 patients, which did not find a significant association between D-dimer levels and mortality (HR = 1.00; 95% CI: 1.00 – 1.00; p-value = 0.583) (25). This difference could be explained by the sample size, as the study by Cidade J. et al. used a relatively small sample of 118 patients, limiting statistical power, unlike the present study, which had a larger sample size. It is also important to consider the sociodemographic, ethnic, and clinical characteristics of the patients, as well as the intervention of potential confounding variables such as the presence of comorbidities and the type of treatment received. There is also the possibility that differences in these results are due to random error. The variability in results underscores the importance of considering the context and specific characteristics of each study. Factors such as sample size and differences in the studied population can influence the strength of the observed association. Replication of studies in different settings is essential to confirm these findings.
The AUC for D-dimer levels at admission, to discriminate between deceased and non-deceased individuals, was 0.92 (95% CI: 0.88 – 0.95; p-value = 0.016). This finding was in line with the study by He X. et al., which determined an AUC of 0.91 for D-dimer to determine the risk of mortality from COVID-19 (25).
Similarly, Zhang et al. (14) demonstrated an AUC of 0.89, while Poudel et al. (21) demonstrated an AUC of 0.81. The high accuracy of D-dimer as a predictor of mortality, reflected in the AUC values, highlights its utility in clinical practice. These results indicate that D-dimer is a valuable tool for risk stratification in hospitalized COVID-19 patients. Implementing this marker could help identify patients at higher risk of mortality early.
The elevation of D-dimer reflects a hypercoagulable state in COVID-19 patients, which can be explained by the aforementioned pathophysiological events and findings in specimens from deceased patients, particularly the presence of microthrombi in small-caliber pulmonary veins, coronary, renal, and hepatic vessels (14, 26). This hypercoagulable state is a distinctive feature of severe COVID-19, significantly contributing to the observed mortality in these patients. Early identification and management of this condition can be crucial to improving clinical outcomes. Continued research in this area is essential to develop more effective therapeutic strategies.
This study has limitations that should be acknowledged. First, the possibility of selection bias and that the sample may not represent the target population for the study due to the retrospective nature of the design, the single-center study setting, and factors related to the quality of medical record documentation, which could compromise the external validity of the study. Another important limitation is that the time from disease onset to hospital presentation was not considered, which could affect D-dimer levels, given the dynamic changes that may occur during that period. Furthermore, elevated D-dimer levels are not specific, so an increase in this variable could be explained by factors related to each patient's individual condition, such as advanced age, chronic bedridden state, the presence of comorbidities, catheter-related thrombosis, the development of disseminated intravascular coagulation, and sepsis-induced coagulopathy. Therefore, the results of this study should be interpreted with caution when making extrapolations to other populations. Prospective studies are needed in the future to elucidate the diagnostic performance of D-dimer in different populations.
CONCLUSION
This study showed that D-dimer levels at admission represent a reliable biomarker in the prognosis evaluation of COVID-19 patients. D-dimer is an easily accessible and low-cost test that should be considered in hospital centers with limitations on certain resources to allow for an accurate assessment of disease severity.
Authorship contributions:
All authors contributed significantly to the completion of the research work.
Financing:
The authors did not receive any financial support for the conduct of this research.
Declaration of conflict of interest:
The authors declare no conflicts of interest regarding the research, authorship, and publication of this research article.
Recevied:
March 15, 2024
Approved:
June 10, 2024
Correspondence author:
Joseph Alburqueque-Melgarejo.
Address:
Callao, Lima, Perú
Phone:
(+51) 979 862 474
E-mail:
jalburqueque@cientifica.edu.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES
