CLINIC CASE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2024 - Universidad Ricardo Palma10.25176/RFMH.v24i3.6605
KAPOSI SARCOMA AND BARTONELLA COINFECTION IN HIV-POSITIVE PATIENT. CASE REPORT
SARCOMA DE KAPOSI Y COINFECCION POR BARTONELLA EN PACIENTE VIH POSITIVO
Roger Antonio Sernaque Mechato
 1,a,b
1,a,b
Clariza Biminchumo Sagastegui
 1,b
1,b
Diego Alejandro Jimenez Mercado
 2,d
2,d
Jesus Dario Toledo De La Torre
 1,c
1,c
1 Hospitsal Santa Rosa. Lima, Perú.
2 Instituto de investigaciones en ciencias biomédicas. Universidad Ricardo Palma, Lima, Perú.
a Internal Medicine.
b Infectology.
c Gastroenterology resident.
d Physician.
ABSTRACT
Introduction: Kaposi's sarcoma is a multifocal malignant neoplasm of endothelial cells, its
etiological agent is HHV-8 and it constitutes one of the defining pathologies of AIDS. It represents
approximately 12% of cancers diagnosed in people living with HIV. Bacillary angiomatosis (AB) is a rare
infectious disease caused by bacteria of the genus Bartonella spp., transmitted by vectors such as
fleas, lice, and mosquitoes. In patients with human immunodeficiency virus (HIV) infection with a CD4+
T-cell count <100 cells/µL, it is associated with angiomatous lesions with neovascularization that
involve the skin and, to a lesser extent, mucous membranes, liver, spleen, and bones.
Clinical case: the case of a 48-year-old male patient with a history of HIV on HAART for 15
years, who was admitted for an outpatient infectious disease clinic due to violaceous nodular lesions in
the right and left MMII, upper eyelid. left and oropharynx. During hospitalization, a blood culture
report was obtained that was positive for Bartonella and a biopsy result of a lower limb lesion
concluded that Kaposi's Sarcoma was present. Upper gastrointestinal endoscopy and chest and abdominal
tomography were performed, which showed the visceral and systemic involvement of Kaposi's Sarcoma. The
HIV genotype is performed, resulting in resistance to antiretrovirals, so the medication is changed and
chemotherapy is started, with the patient showing a good response and improvement.
Conclusion: HIV-related Kaposi's Sarcoma affects AIDS patients in a much more severe,
aggressive, and fulminant manner compared to other immunodeficient groups. However, when presenting
characteristic lesions, we must consider its main differential diagnosis: Bacillary Angiomatosis, which,
even very uncommonly, may occur simultaneously.
Keywords: Kaposi sarcoma, HIV, AIDS, Bacillary angiomatosis. (source: MeSH NLM)
RESUMEN
Introducción: El Sarcoma de Kaposi es una neoplasia maligna multifocal de células endoteliales.
Su agente etiológico es el HHV-8 y constituye una de las patologías definitorias de SIDA. Representa
aproximadamente el 12% de los cánceres diagnosticados en personas que viven con VIH. La angiomatosis
bacilar (AB) es una enfermedad infecciosa poco frecuente, causada por bacterias del género Bartonella
spp. transmitidas por vectores como pulgas, piojos y mosquitos. En pacientes con infección por el virus
de inmunodeficiencia humana (VIH) con recuento de LT CD4 + <100 cél/µL se asocia a lesiones
angiomatosas con neovascularización que comprometen la piel y, en menor medida, mucosas, hígado, bazo y
huesos.
Caso clínico: Paciente varón de 48 años con antecedente de VIH en TARGA hace 15 años, que ingresa
por consulta externa de infectología debido a lesiones nodulares violáceas en MMII derecho, izquierdo,
parpado superior izquierdo y orofaringe. Durante hospitalización se obtiene hemocultivo positivo para
Bartonella y resultado de biopsia de lesión de miembro inferior que concluye Sarcoma de Kaposi. Se
indica endoscopia digestiva alta y tomografía de tórax y abdomen que evidencian compromiso visceral y
sistémico. Se realiza genotipo VIH resultando resistencia a antirretrovirales por lo que se rota
medicamentos y se inicia quimioterapia presentando buena respuesta y mejoría.
Conclusión: El Sarcoma de Kaposi relacionado al VIH afecta a pacientes con SIDA de una forma
mucho más severa, agresiva y fulminante en comparación con otros grupos de pacientes inmodeficientes.
Sin embargo, al presentar lesiones características, debemos tener en cuenta su principal diagnóstico
diferencial: Angiomatosis Bacilar, que incluso, de manera muy poco común, pueden presentarse en
simultáneo.
Palabras clave: Sarcoma de Kaposi, VIH, SIDA, Angiomatosis bacilar. (fuente: DeCS-BIREME)
INTRODUCTION
Without treatment, HIV infection causes AIDS-related cancers such as non-Hodgkin's Lymphoma, Cervical
Cancer, and Kaposi's Sarcoma (1, 2).
Kaposi's sarcoma represents approximately 12% of cancers diagnosed in people living with HIV. (3) Seroprevalence varies geographically between continents, being highest in
regions such as Africa and Latin America and less prevalent in Europe and North America (4). Furthermore, it has a high prevalence among men who have sex with men,
particularly in those with HIV co-infection. It is a multifocal malignant neoplasm of endothelial cells
first described in 1872 by Moritz Kaposi. The risk of presenting in this population is 500 times greater
(4).
Four types of Kaposi Sarcoma have been described with different epidemiological and clinical
characteristics (1); the classic, endemic, iatrogenic and the epidemic that
occurs in the context of HIV. It has been universally associated with HHV-8 infection; its presence is
considered a primary and necessary factor for its development (5).
The question of whether Kaposi sarcoma is a clonal neoplasm, oligoclonal process, or multifocal vascular
proliferation has not yet been completely resolved. Recent studies are consistent with Kaposi's Sarcoma
being a multifocal disease in which individual lesions arise separately rather than by metastasis.
However, additional research is needed. Whatever the resolution, from a clinical perspective,
HIV-associated Kaposi's sarcoma acts like other neoplastic processes with invasion of several organs and
devastating results if left untreated (6).
Clinically, Kaposi's sarcoma presents with asymptomatic violet-pink or red macular skin lesions that can
converge to form violet-blue or black plaques and nodules that are frequently associated with edema in
the lower extremities (7). Patients may develop woody edema that may persist
even after treatment and long-standing edema may lead to recurrent skin infections and mobility problems
(6). These skin lesions often spread. In severe cases, lesions may occur in
the oral cavity as nodular lesions, lymph nodes or any visceral organ, most commonly the
gastrointestinal tract or pulmonary system. In some cases, bone lesions are observed on images that may
be intermittently symptomatic.
For patients with respiratory or gastrointestinal symptoms, bronchoscopy or endoscopy may be beneficial
to visualize lesions and stage disease. CT scan is useful in showing visceral Kaposi sarcoma and
determining the stage of the disease. Biopsies of the skin or gastrointestinal system should be obtained
to confirm the diagnosis, but biopsy of the respiratory tract is not recommended. Diagnostic features of
Kaposi sarcoma on biopsy include the presence of endothelial markers, spindle cells, and positive
staining for nuclear antigens associated with HHV-8 latency on immunohistochemistry(6).
Kaposi sarcoma and bacillary angiomatosis are the main differential diagnoses in patients with HIV and
low CD4 lymphocyte count. Both diseases present similar skin manifestations, which makes them clinically
indistinguishable (8). The responsible species can be suspected based on
epidemiological risk factors and predilection for some organ. Bartonella Quintana infection is found in
patients with low socioeconomic status, chronic alcoholism and corporal pediculosis, and its clinical
manifestations are associated with bacillary angiomatosis, bone and subcutaneous involvement.
Nevertheless, Bartonella Henselae, associated with cat bites or scratches and flea bites, mainly causes
cat scratch disease (CSD) with regional lymphadenopathy and extra-nodal involvement, mainly related to
hepatic peliosis. Histopathologically, both diseases are characterized by proliferative vascular lesions
that manifest as small papules that can evolve into friable, purplish-red nodules, with various shapes
such as nodular, peduncular or warty(8).
The presence of coinfection is uncommon, much less dual or multiple pathological processes in the skin,
however, they may exist(9).
CLINICAL CASE
The case of a 48-year-old male patient from Tumbes with a history of HIV treated with HAART with
Tenofovir, Lamivudine and Efavirenz since 2009, Pneumocystosis in 2009, HBV, psoriasis, HTN and
appendectomy is presented. He reported a period of illness of approximately 13 months when he presented
a single 1 cm dark and pruritic punctate plaque-type lesion in the right lower limb that evolved into
lesions in both lower limbs. Seven days before the onset of the injuries, he had a bite in the
supracondylar region of the right lower limb that he reported as “infected,” so he went to the Tumbes
Hospital where he received antibiotic treatment. Eight months later, he presented non-pruritic dark
violaceous plaque-like lesions with irregular edges measuring 3cm on the dorsum of his right foot that
progressed to nodular lesions. Four months before admission to our hospital, he showed the same lesions
in the groin and shaft of the penis adjacent to the foreskin. A few weeks later he noticed an increase
in volume and pruritic erythema on the left upper eyelid that progressed to a violaceous nodular lesion
measuring approximately 1cm and a sensation of a progressively growing foreign body in the oropharynx.
Subsequently, he presented chills with unquantified feverish peaks associated with cough and nasal
congestion that progressed to dyspnea, which is why he went to the emergency room where, after
evaluation and treatment, an evaluation by Infectology was suggested, who requested evaluation by
oncology and tests to rule out Bartonella infection. Infectology evaluates with a verbal blood culture
report that indicates Bartonellosis and suggests hospitalization to manage the infection.
Physical examination upon admission: Vital functions: BP 100/70, HR 88 x´, RR 16x´, T 36.5 ºC, SatO2
98%, Head and neck: Normocephalic, cylindrical neck. Skin and skin: warm, elastic capillary refill for 2
seconds, mild, non-painful lymphadenopathy in the maxillofacial region. Violet nodules with defined
edges and semi-soft consistency in the groin, right buttock. Chest and lungs: Rhythmic heart sounds of
good intensity, without murmurs, the vesicular murmur passes well in both lung fields, without added
noises. Abdomen Globose, hydroaerial sounds(+) soft, depressible, not painful on palpation.
Neurological: oriented to place, time, space and person, Glagow Scale: 15 points, without meningeal
signs.
In the plantar region (Figure 1), hyperpigmented nodules with defined edges of hard, scaly consistency
can be seen distributed in the middle 1/3 plantar region and part of the lower part in MMII I, middle
1/3 in right MMII. In the left palpebral region (Figure 2a), a semi-soft violaceous hyperpigmented
multinodular formation can be seen; Likewise, a lesion with similar characteristics located in the
midline without inflammatory signs is also seen in the oropharynx region (Figure 2b).
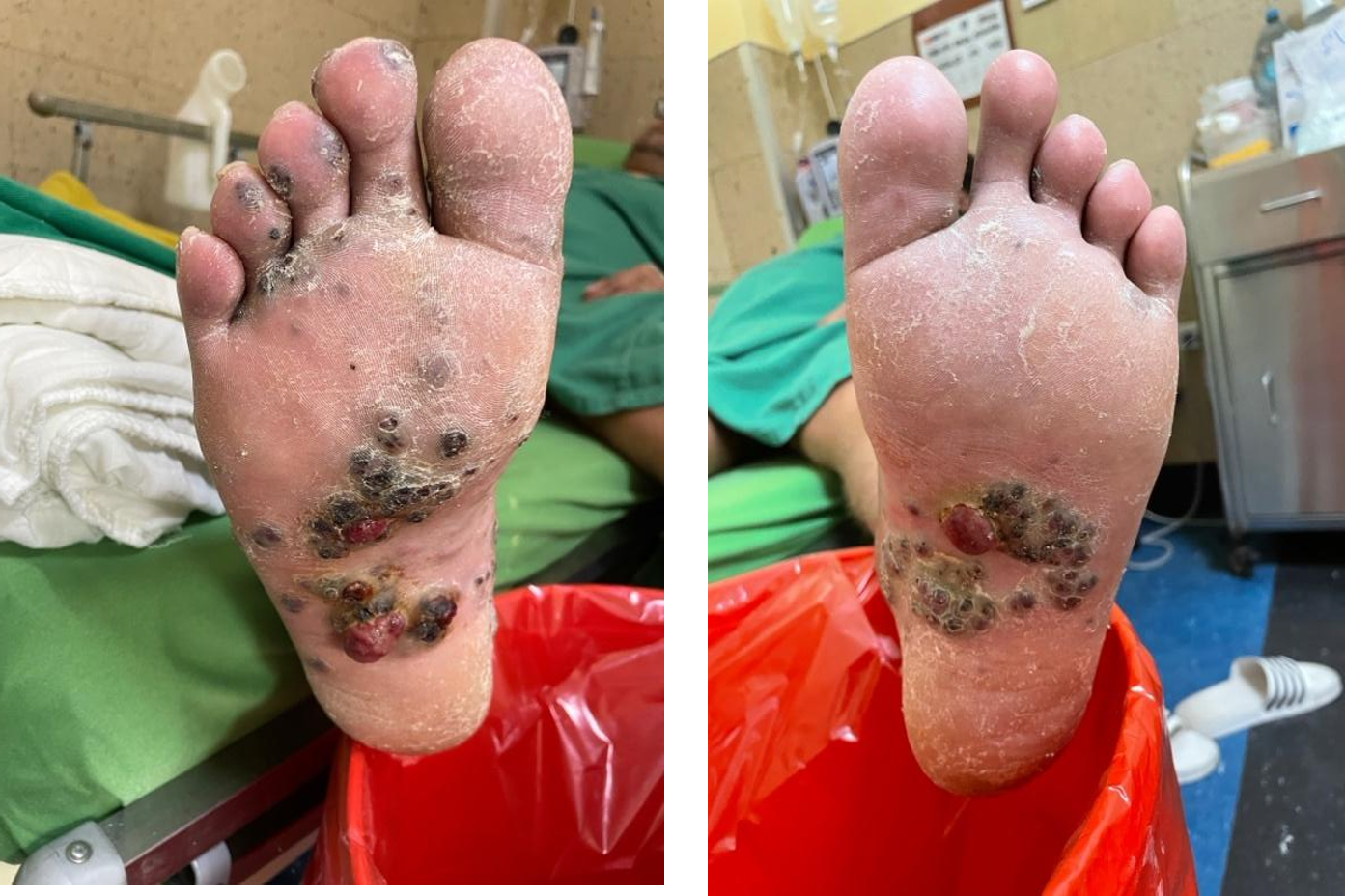
Figure 1: violet-blue or black nodular lesions on the right and left lower limb
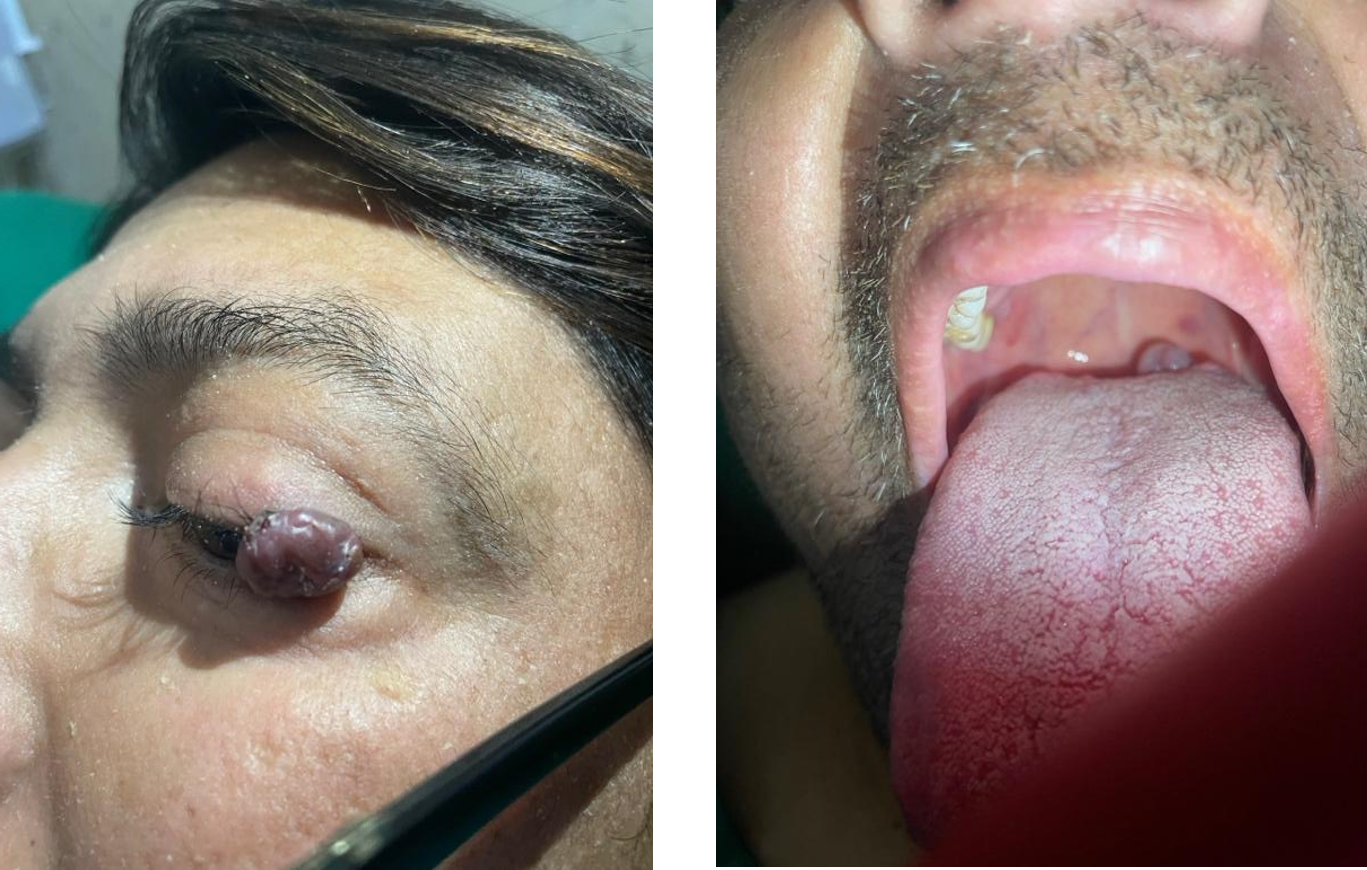
Figure 2a) purple multinodular lesion in left eyelid. 2b) purple multinodular lesion in the oral cavity
Laboratory tests: Leukocytes 11150, Segmented 69, Lymphocytes 22, Batoned 0, eosinophils 2, Red blood
cells 4880000, Hb 13.9, Hematocrit 42, HCM 29, CHCM 33, VCM 87, Platelets 365000, Glucose 88, Urea 45,
TGO 25 , TGP 37, Creatinine 0.85. Viral load 387000. VDRL Reagent 8 dilutions, Syphilis IgG IgM Reagent,
Total Anti HBc Reagent. Biopsy of a nodular lesion on the lower limb brought by the patient, performed
in another hospital a month before admission, reports Kaposi's Sarcoma. Deep edge engaged.
During hospitalization, a report was obtained from the Institute of Tropical Medicine that reported
positive coccobacillary forms in Gram blood culture for Bartonella (Figure 3). An upper digestive
endoscopy was performed, which reported Kaposi's sarcoma in the gastric antrum and esophagus, gastric
ulcers, and antral erythematous gastritis. A CT scan of the abdomen and pelvis was also performed, which
reported inflammatory-looking retroperitoneal lymphadenopathy of up to 10 mm in short axis and multiple
mesenteric lymph nodes measuring 10-11 mm in short axis, most with fatty hilum present. Chest CT c/c
(Figure 4) reports scattered nodules, the largest measuring 13x8 mm with a peribronchovascular
distribution in the left upper lobe with “flame” morphology and other smaller ones in both upper lobes
and bases, surrounded by ground glass halos and fibroatelectasis. in lingula.

Figure 3: Blood culture result
Figure 3: c/c chest CT
Based on the patient's clinical manifestations, biopsy results, blood culture and procedures performed,
it is concluded that the patient has the following diagnoses: Systemic or disseminated Kaposi's sarcoma
with visceral and cutaneous involvement, Bacteremia due to Bartonella spp, HIV infection, AIDS stage
with HAART failure, undetermined Latent Syphilis and Hepatitis B. Therefore, treatment with
Ciprofloxacin, Ceftriaxone, Azithromycin and Benzathine Penicillin is indicated. Dermatology requests
PCR, culture and serology for Bartonella quintana and Bartonella baciliformes. Infectology suggests
evaluation by oncology for the initiation of chemotherapy because there is systemic involvement and
progression of the disease could affect the administration of antiretrovirals, which are oral. Oncology
indicates that the patient is not eligible for treatment with chemotherapy due to viral load, requests
CD4 and infection control, for this reason a medical meeting is held where it is concluded to continue
HAART previous regimen until obtaining Genotype results and medical discharge for outpatient control by
medical oncology to start chemotherapy.
Subsequently, ELISA results were obtained for IgG and IgM for Carrión's Disease, which reported
non-reactive, and by indirect immunofluorescence IgG and IgM for Bartonella hanselae were negative. They
did not perform serology or the requested Bartonella quintana PCR. ELISA Anti total HBc, HBeAg, Anti HBe
and non-reactive Anti HBs. The HIV genotype reported a high level of resistance to Efavirenz, so HAART
was started with a new regimen of Tenofovir, Lamivudine and Dolutegravir, presenting an undetectable
viral load 2 months later. The patient completed 16 cycles of chemotherapy with Paclitaxel, showing a
good response and obvious improvement.
DISCUSSION
In recent years, the epidemiology and prognosis of HIV infection have experienced significant changes
thanks to antiretroviral treatment for infected people, the development of more effective and better
tolerated drugs, and preventive measures such as pre-exposure prophylaxis. The evolution of
antiretroviral therapy, now with simple oral and injectable options, has also contributed to the
improvement of comprehensive HIV treatment and care. With early diagnoses and early initiation of
antiretroviral therapy, the life expectancy of people with HIV has become equal to that of the general
population. However, many people remain undiagnosed or are diagnosed late and there are population
groups subjected to situations of greater vulnerability that affect individual and collective health.
Kaposi's sarcoma is considered the neoplasm most frequently associated with HIV, affecting patients with
AIDS in a much more severe, aggressive and fulminant manner compared to other groups of immunodeficient
patients. Its diagnosis is mainly clinical, characterized by the presence of macules that progress to
violet or brown nodules(4). Procedures such as endoscopy and tomography are
useful to visualize the lesions if there is visceral involvement, as well as to determine the stage of
the disease, but when there is doubt, diagnose with bacillary angiomatosis because clinically they can
be indistinguishable(7, 8), as occurred in this case, the
use The biopsy, which is the Gold standard for diagnosis, is very useful and allows confirming the
diagnosis; without failing to take into account that both conditions can occur concomitantly in very
isolated cases.
The emergence of strains resistant to antiretroviral treatment may depend on different factors of both
the host and the virus(6). Non-adherence to treatment is the main cause of
virological failure and has been strongly associated with the emergence of specific resistance mutations
for different classes of drugs(8). In our clinical case presented, it is
observed that the patient had been on treatment with HAART (Highly Active Antiretroviral Therapy) since
2009, which indicates that he has been receiving treatment for his HIV infection for a prolonged
period(10). However, the patient was identified as having a high level of
resistance to Efavirenz, one of the medications used in his previous HAART regimen. The change to a new
treatment regimen with Tenofovir, Lamivudine and Dolutegravir seems to have been effective, since two
months after starting, the patient showed an undetectable viral load.
Reduction of immunosuppression can cause remission of Kaposi's sarcoma, highlighting the fundamental
role of the immune response in this infection. Similarly, epidemic or HIV-related Kaposi Sarcoma usually
responds to stimulation of the immune response with antiretroviral therapy(9). Epidemic Kaposi sarcoma is usually aggressive and especially affects the
skin, digestive system and respiratory system. Pulmonary Kaposi Sarcoma usually produces lesions in the
bronchial mucosa, but can be associated with various radiographic manifestations such as nodules,
lymphadenopathy and pleural effusions. Unlike the lesions of classic Kaposi's sarcoma that affects the
elderly and appears on the skin of the lower extremities, those of epidemic Kaposi's sarcoma usually
affect the face, frequently the nose, genitals and oral cavity (palate and gums), in addition of the
lower extremities (1). The clinical case presents similarities with the
typical presentation of HIV-related epidemic Kaposi Sarcoma, including the distribution of skin lesions
and the involvement of other systems, but does not provide specific information on the remission of
Kaposi Sarcoma with reduction of the immunosuppression. However, the response to the change in
antiretroviral treatment and chemotherapy suggests an improvement in disease control.
Kaposi's sarcoma manifests itself in the form of lesions that increase in size, changing from spots to
plaques and later to nodules. The lesions are usually purplish in color at first and then turn brown due
to the deposition of hemosiderin. Kaposi sarcoma lesions are composed of vascular spaces, extravasated
erythrocytes and different types of cells, including malignant spindle cells and infiltrating
mononuclear cells such as hemosiderin-laden macrophages. The hypervascular nature gives it its purple
color. In the nodular stage, almost all spindle cells are infected by HHV-8 (1). In the clinical case, the lesions evolve to plaques and then to
violaceous nodules in the lower limbs, genitals, and other areas. The progression of our clinical case
agrees with the classic description of Kaposi's Sarcoma. Histologically, the lesions show vascular
spaces, extravasated erythrocytes and malignant spindle cells. HHV-8 infection is associated,
highlighting the viral nature of Kaposi's Sarcoma. Treatment included chemotherapy and change of
antiretroviral regimen due to resistance.
Both Kaposi's Sarcoma and Bartonella occur in HIV-infected patients with low LT CD4 counts. Bartonella
can have oral lesions that mimic those of sarcoma. Although an experienced clinician usually recognizes
Kaposi's sarcoma, the biopsy easily confirms the diagnosis, which is why the anatomopathological study
is essential for the definitive diagnosis and thus differentiates Kaposi's sarcoma from bacillary
angiomatosis (9). This is why it is necessary to apply the auxiliary
histopathological examinations together, for the adequate support of the pathology provided.
CONCLUSION
The differential diagnosis of Kaposi's sarcoma should be made with bacillary angiomatosis, which is
caused by Bartonella species. The presence of dual or multiple pathological processes in a skin biopsy
is uncommon, in addition to which the existence of two diseases in the same biopsy sample can be
overlooked, especially when there is histological predominance of one type of lesion. . For this reason,
it is very important to maintain a high index of clinical suspicion in severely immunosuppressed
HIV/AIDS patients without HAART.
The treatment of Kaposi's Sarcoma in HIV infection is palliative, but not curative. Depending on the
severity of the disease, therapeutic options may include combined antiretroviral therapy as the first
line of treatment in the cutaneous manifestation and in case of visceral involvement or rapidly
progressive disease, chemotherapy is indicated in conjunction with antiretroviral therapy. In the case
presented, since there was evident treatment failure, it was necessary to carry out an HIV genotype
study, with the results resistance to antiretrovirals was determined, for which the antiretroviral
medications were changed and since the patient had visceral involvement, he benefited from the use of
chemotherapy plus HAART adjustment resulting in evident improvement.
Authorship contributions:
The authors have participated in the conception of the article, search for information,
writing and approval of the final version.
Financing:
Self-funded
Declaration of conflict of interest:
The authors declare that they have no conflict of interest.
Recevied:
March 14, 2023
Approved:
June 12, 2024
Correspondence author:
Roger Sernaque Mechato.
Address:
Hospital Santa Rosa, Lima Perú.
Phone:
(+51) 998995740
E-mail:
internistagg@gmail.com
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES
