ORIGINAL ARTICLE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2024 - Universidad Ricardo Palma10.25176/RFMH.v24i3.6679
IMPACT OF NEUTROPHIL-LYMPHOCYTE RATIO IN ACRAL LENTIGINOUS MELANOMA
IMPACTO DEL ÍNDICE NEUTRÓFILO/LINFOCITO EN EL MELANOMA LENTIGINOSO ACRAL
Joseph Alburqueque-Melgarejo
 1,a
1,a
Martha Eugenia Aguirre Coronado
 2,b
2,b
Brady Beltrán Gárate
 3,c
3,c
1 Universidad Cientíca del Sur, Lima, Peru.
2 Hospital de Emergencias José Casimiro Ulloa, Lima, Peru.
3 Instituto de investigaciones en Ciencias Biomédicas. Faculty of Human Medicine. Universidad
Ricardo Palma, Lima, Peru.
a Master in Medicine
b Internal Medicine
c Medical Oncologist
ABSTRACT
Introduction: Acral lentiginous melanoma (ALM) is the fourth type of cutaneous melanoma and is
the most common subtype in some countries in Latin America and Asia. The neutrophil-lymphocyte ratio
(NLR) is an inflammatory marker that has been shown to be useful as a prognostic tool in several
malignant neoplasms.
Objective: The objective of the study was to evaluate whether NLR has prognostic value in ALM. A
retrospective study was conducted that included patients with ALM between 2010 and 2015.
Methods: An observational, analytical and retrospective cohort design was used. We worked with a
total population of 69 patients with the diagnosis of acral lentiginous melanoma. For the statistical
analysis, the SPSS statistical package version 26 was used. Univariate and multivariate Cox proportional
regression models were performed.
Results: A total of 69 patients with ALM were included. The median age was 68 years, with a
predominance of females (55%). Most patients had T4 (34%), lymph node involvement (57.1%), and Clark III
(34.4%). In univariate analysis, Clark level III/IV, anaplasia, lymphocytic infiltration, stage III-IV,
and NLR were associated with prognoses. In the multivariate analysis, NLR >3.5 (HR 3.9, 95% CI 1.5-10.3,
p=0.005) and Clark level III-IV (HR 3.5, 95% CI 1.6-7.8, p= 0.002) were associated with poor overall
survival (OS).
Conclusions: NLR is an independent prognostic factor for survival in ALM.
Key words (MeSH): Acral Lentiginous melanoma, Prognostic factor, Survival, Neutrophil-Lymphocyte
Ratio.
RESUMEN
Introducción: El melanoma lentiginoso acral (MLA) es el cuarto tipo de melanoma cutáneo y es el
subtipo más común en algunos países de América Latina y Asia. El índice neutrófilo-linfocito (NLR) es un
marcador inflamatorio que ha demostrado tener utilidad como herramienta pronóstica en varias neoplasias
malignas.
Objetivo: El objetivo del estudio fue evaluar si el NLR tiene valor pronóstico en la MLA. Se
realizó un estudio retrospectivo que incluyó pacientes con MLA entre 2010 y 2015.
Métodos: Se empleó un diseño observacional, analítico, y de tipo cohorte retrospectiva. Se
trabajó con una población total de 69 pacientes con el diagnóstico de melanoma acral lentiginoso. Para
el análisis estadístico se empleó el paquete estadístico SPSS versión 26. Se realizaron modelos de
regresión proporcional de Cox univariados y multivariados.
Resultados: Se incluyeron un total de 69 pacientes con MLA. La mediana de edad fue 68 años, con
predominio del sexo femenino (55%). La mayoría de los pacientes tenían T4 (34%), compromiso ganglionar
(57,1%) y Clark III (34,4%). En el análisis univariado, el nivel de Clark III/IV, la anaplasia, la
infiltración linfocitaria, el estadio III-IV y el NLR se asociaron con el pronóstico. En el análisis
multivariado, el NLR >3,5 (HR 3,9, IC 95% 1,5-10,3, p=0,005) y el nivel de Clark III-IV (HR 3,5, IC 95%
1,6-7,8, p= 0,002) se asociaron con mala supervivencia general (SG).
Conclusiones: El NLR es un factor pronóstico independiente de supervivencia en la MLA.
Palabras clave (DeCS): Melanoma Acral Lentiginoso, Factor pronóstico, Sobrevida, Radio
Neutrófilo-Linfocito.
Introduction
Malignant melanoma (MM) is the deadliest form of skin cancer worldwide(1). According to GLOBOCAN 2018, MM ranks thirteenth in global prevalence, with the highest number of cases reported in the United States, Germany, the United Kingdom, and Australia(2, 3). The main histological subtypes of cutaneous melanoma are superficial spreading melanoma, nodular melanoma, lentigo maligna, and acral melanoma. Acral melanoma is defined by its location and was first described by Reed in 1976, with the most common histological subtype being acral lentiginous melanoma (ALM)(4).
While the genesis of non-acral melanoma is related to intermittent sun exposure, the pathogenesis of ALM is not fully defined(1). Some studies have associated it with traumatic injuries, ultraviolet light exposure, and chemical exposure as risk factors, while others do not find these associations(5-8). Clinically relevant prognostic factors for malignant cutaneous melanoma include tumor depth, clear margins, sentinel lymph node biopsy, and ulceration. Mitosis count is no longer considered relevant for prognosis. For the ALM subtype, clinically relevant prognostic factors include tumor depth and clinical stage(9).
From a pathophysiological perspective, the use of NLR (neutrophil-to-lymphocyte ratio) is supported by the roles of both cell populations. Neutrophils, with their pro-tumoral phenotype, promote tumor growth, angiogenesis, and invasion, while lymphocytes, particularly T lymphocytes, play a crucial role in immune surveillance and response against cancer. An elevated NLR suggests a pro-inflammatory environment that may favor tumor progression and exhibit an ineffective immune response against cancer. Recently, new prognostic parameters such as the NLR have emerged, widely used in other types of neoplasms. We previously reported that NLR is related to survival in patients with aggressive B-cell and T-cell lymphomas(10, 11).
The clinical relevance of using NLR in Peruvian patients with ALM lies in its non-invasive and easily accessible nature, allowing for early prognosis estimation and risk stratification. Therefore, this study aims to demonstrate the prognostic value of NLR in patients with ALM, the most common cutaneous melanoma in Peru.
MethodsStudy Design and Area
This study employed a quantitative, observational, retrospective cohort design. The STROBE checklist for cohort studies was applied(12).
Population and SampleThe study population consisted of patients diagnosed with Acral Lentiginous Melanoma treated at the Hospital Edgardo Rebagliati Martins between 2010 and 2015. Inclusion criteria considered patients with a histopathological diagnosis of Acral Lentiginous Melanoma, age over 18 years, complete clinical information, and adequate follow-up. Patients with a second neoplasm and those with incomplete clinical information were excluded.
Variables and InstrumentsData for the study variables were collected from patients' medical records.
Patients' clinical characteristics were classified accordingly.
The gender variable was assessed as a dichotomous qualitative variable. The primary tumor site was
defined as a polytomous qualitative variable. Age was evaluated as a continuous quantitative variable
and later divided into a dichotomous qualitative variable based on whether patients were considered
elderly.
Tumor pathological characteristics were evaluated as qualitative variables, including Clark levels
(I-IV), Breslow thickness (0.01-1, 1.01-2, 2.01-4, >4), presence of anaplasia (present, absent,
unknown), ulceration (present, absent, unknown), microsatellitosis (present, absent, unknown),
perineural infiltration (present, absent, unknown), lymphocytic infiltration (present, absent, unknown),
vascular invasion (present, absent, unknown), nodal involvement (present, absent, unknown), number of
affected nodes (1-2, 3-5, >6), pathological stage according to the 7th edition of the American Joint
Committee on Cancer (AJCC 7th edition) (I, II, III, IV, unknown).
The neutrophil-to-lymphocyte ratio (NLR) was defined as the ratio of absolute neutrophil count to
absolute lymphocyte count (NLR = ANC/ALC). Hematological variables (absolute counts of neutrophils,
lymphocytes, monocytes, platelets) were measured using a Sysmex XN-1000 hematology analyzer, employing
flow cytometry. Overall survival (OS) was calculated from the diagnosis of the disease (event) to the
date of the last follow-up (censored).
Data collected were entered into a database using SPSS version 26 for statistical analysis. Clinical-pathological information was analyzed using descriptive statistical tools, including absolute and relative frequencies for qualitative variables. An NLR cutoff of 3.5 was used for patient stratification according to the study by Vano Y et al.(13). Inferential analysis employed survival analysis using the Kaplan-Meier method to generate survival curves, compared using the log-rank test. Additionally, a Cox proportional hazards regression model was conducted to establish univariate and multivariate survival models. The results of the Cox proportional hazards regression model were reported with a hazard ratio (HR) and their respective 95% confidence intervals (CIs). A p-value was considered significant if it was less than 0.05.
Ethical AspectsThe research project was approved (Letter N° 387-GRPR ESSALUD-2023) by the Ethics Committee of the Hospital Edgardo Rebagliati Martins. Patient confidentiality and rights were respected according to bioethical principles and the Declaration of Helsinki.
ResultsA total of 69 patients were analyzed, with a significant predominance of females (51.1%). The mean age was 68 years, and 68% of patients were over 60 years old. The plantar location was the most frequently affected site in ALM, with 88.4%, followed by palmar location at 8.6%, and subungual location at only 2.9% of cases (Table 1).
|
N |
% |
|
|---|---|---|
| Patients | 69 | |
| Age | ||
| Median (IQR) | 68 (16, 89) | |
| <60 | 22 | 31.9 |
| >60 | 47 | 68.1 |
| Gender | ||
| Female | 38 | 55.1 |
| Male | 31 | 44.9 |
| Primary site | ||
| Plantar | 61 | 88.4 |
| Palmar | 6 | 8.6 |
| Subungual | 2 | 2.9 |
*IQR: Interquartile Range
In relation to the pathological characteristics, it was found that a Breslow thickness between 0.01 and 1 mm was present in 18.8% of the patients, 1.01-2 mm in 21.7%, 2.01-4 mm in 13% and more than 4 mm in 37.7% of patients. Ulceration was absent in more than half of the patients (58.0%) and most patients did not have perineural infiltration, lymphocyte infiltration and vascular invasion (89.9%, 82.6%, and 89.9%, respectively). Anaplasia and microsatellitosis were present in 5.8% and 1.4%, respectively. No tumor regression was found. Nodal involvement was present in 17.4%. Pathological stage I was present in 28.9% of the patients, stage II in 34.8%, stage III in 24.6%, and stage IV in 5.8% (Table 2).
|
N |
% |
|
|---|---|---|
| Patients | 69 | |
| Clark levels | ||
| I | 7 | 10.1 |
| II | 14 | 20.3 |
| III | 21 | 30.4 |
| IV | 13 | 18.8 |
| V | 6 | 8.7 |
| Unknown | 8 | 11.6 |
| Breslow | ||
| 0.01-1 | 13 | 18.8 |
| 1.01-2 | 15 | 21.7 |
| 2.01-4 | 9 | 13.0 |
| > 4 | 26 | 37.7 |
| Unknown | 6 | 8.7 |
| Anaplasia | ||
| No | 59 | 85.5 |
| Yes | 4 | 5.8 |
| Unknown | 6 | 8.7 |
| Ulceration | ||
| No | 40 | 58.0 |
| Yes | 25 | 36.2 |
| Unknown | 4 | 5.8 |
| Microsatellitosis | ||
| No | 64 | 92.8 |
| Yes | 1 | 1.4 |
| Unknown | 4 | 5.8 |
| Perineural infiltration | ||
| No | 62 | 89.9 |
| Yes | 3 | 4.3 |
| Unknown | 4 | 5.8 |
| Lymphocytic infiltration | ||
| No | 57 | 82.6 |
| Yes | 8 | 11.6 |
| Unknown | 4 | 5.8 |
| Vascular invasion | ||
| No | 62 | 89.9 |
| Yes | 3 | 4.3 |
| Unknown | 4 | 5.8 |
| Nodal involvement | ||
| No | 53 | 76.8 |
| Yes | 12 | 17.4 |
| Unknown | 4 | 5.8 |
| N° positive lymph nodes | ||
| 1-2 | 7 | 58.3 |
| 3-5 | 4 | 33.3 |
| >6 | 1 | 8.3 |
| Regression | ||
| No | 65 | 94.2 |
| Yes | 0 | 0.0 |
| Unknown | 4 | 5.8 |
| Stage (7th Edition AJCC) | ||
| I | 20 | 28.9 |
| II | 24 | 34.8 |
| III | 17 | 24.6 |
| IV | 4 | 5.8 |
| Unknown | 4 | 5.8 |
*AJCC: American Joint Committee on Cancer
82.6% of patients with clinical stage I to III underwent surgical intervention, and 76.9% of the patients had a complete lymph node dissection (CLND). Adjuvant treatment was performed in 32.3% of patients with stage IIB to IIIC. All patients with stage IV received the best supportive care and no systemic treatment due to poor functional status. The overall 5-year survival rate was 54.3% with a median of 6.3 months.
Univariate analysis using a Cox proportional hazards regression model showed that the variables Clark levels IV-V (HR: 1.8, 95% CI, 1.1-3.2, p = 0.016), anaplasia (HR: 3.0, 95% CI, 1.5-5.7, p = 0.022), lymphocyte infiltration (HR: 2.8, 95% CI, 1.6-5.0, p = 0.035), advanced clinical stage (HR: 2.5, 95% CI, 1.5-4.1, p = 0.030), and NLR > 3.5 (HR 2.1; 95% CI, 1.1-4.19, p = 0.002) were associated with poor prognosis. (Table 3).
| Median | 5-year OS (%) | HR | p-value | |
|---|---|---|---|---|
|
Overall Survival |
63 |
54.3 |
||
| Age | ||||
| <60 | NR | 52.8 | Reference | empty |
| >60 | 5.4 | 55.1 | 1.2 (0.7, 2.1) | 0.867 |
| Sex | ||||
| Female | 6.8 | 63.6 | Reference | |
| Male | 3.5 | 45.1 | 2.1 (1.3, 3.5) | 0.130 |
| Clark Levels | ||||
| I-III | 6.7 | 58.7 | Reference | |
| IV-V | 2.5 | 32.3 | 1.8 (1.1, 3.2) | 0.016 |
| Breslow Thickness | ||||
| <1 | 6.3 | 54.3 | Reference | |
| >1 | 4.4 | 42.0 | 1.2 (0.6, 2.4) | 0.644 |
| Anaplasia | ||||
| No | 5.4 | 53.6 | Reference | |
| Yes | 0.4 | 25.0 | 3.0 (1.5, 5.7) | 0.022 |
| Ulceration | ||||
| No | 6.3 | 61.1 | Reference | |
| Yes | 3.2 | 41.8 | 1.8 (1.1, 3.0) | 0.453 |
| Lymphocytic Infiltration | ||||
| No | 6.3 | 57.4 | Reference | |
| Yes | 2.2 | 25.0 | 2.8 (1.6, 5.0) | 0.035 |
| Clinical Stage | ||||
| I-II | 6.7 | 65.2 | Reference | |
| III-IV | 2.5 | 30.9 | 2.5 (1.5, 4.1) | 0.030 |
| NLR | ||||
| <3.5 | 6.6 | 57.6 | Reference | |
| >3.5 | 1.7 | 31.7 | 2.1 (1.1, 4.19) | 0.002 |
| LMR | ||||
| >0.2 | 6.8 | 62.5 | Reference | |
| <0.2 | 4.4 | 49.6 | 1.7 (1.0, 3.1) | 0.064 |
| PLR | ||||
| <170 | 6.6 | 58.7 | Reference | |
| >170 | 4.3 | 43.6 | 2.1 (1.2, 3.4) | 0.085 |
*OS: Overall Survival, NLR: Neutrophil-Lymphocyte Ratio, LMR: Lymphocyte-Monocyte Ratio, PLR: Platelet-Lymphocyte Ratio
The multivariate analysis using a Cox proportional hazards regression model showed that the variables Clark levels IV-V (HR 3.5; 95% CI, 1.6-7.8, p = 0.002) and NLR >3.5 were associated with lower overall survival (HR: 3.9, 95% CI, 1.5-10.3, p = 0.005) (Table 4).
| 95% CI | |||||
|---|---|---|---|---|---|
| p-value | HR | Lowe | Upper | ||
| Clark Level | |||||
| I-III | Reference | ||||
| IV-V | 1.2 | 0.002 | 3.5 | 1.6 | 7.8 |
| NLR | |||||
| <3.5 | Reference | ||||
| >3.5 | 1.4 | 0.005 | 3.9 | 1.5 | 10.3 |
*HR: Hazard Ratio, 95% CI: 95% Confidence Interval
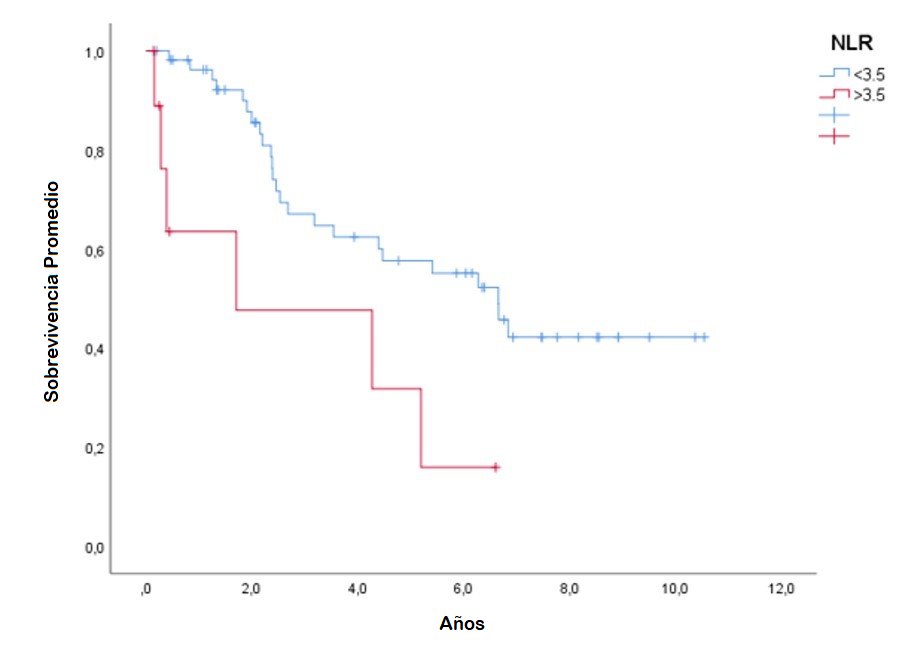
The Kaplan-Meier survival curves for NLR levels according to the cutoff point of 3.5 to evaluate overall survival in patients diagnosed with acral lentiginous melanoma showed statistically significant differences between the high NLR and low NLR groups, according to the log-rank test (p<0.001). (Figure 1)
Figure 1: Kaplan-Meier curve for NLR with a cutoff point of 3.5 to evaluate prognosis in patients diagnosed with Acral Lentiginous Melanoma
Discussion
This is the first report in Latin America to study the role of NLR in ALM. In our cohort of 135 patients with cutaneous melanoma, 51.1% had ALM (69 cases). A previous Peruvian study found that ALM is the most frequent subtype of melanoma, accounting for 61.2% compared to non-acral melanomas(14).
In non-acral cutaneous melanoma, some studies demonstrate the prognostic value of NLR, and three meta-analyses have confirmed its predictive role with checkpoint inhibitors or anti-BRAF treatment(15, 16, 17). Recently, Cocorocchio et al. found that clinical stage, elevated serum LDH, and NLR > 5 were prognostic of OS in patients with advanced melanoma receiving BRAFi alone or combined with a MEK inhibitor (MEKi) at recommended doses. These data suggest an immunomodulatory effect of the therapy on the tumor microenvironment in addition to the direct treatment effect and could indicate some potential clinical biomarkers(18). Finally, Bartlett et al. conducted a prospective study with metastatic cutaneous melanoma patients receiving anti-PD1 treatment, describing that patients with NLR > 5 had a higher disease burden and poorer functional status, which correlated with worse OS in both univariate and multivariate analyses(19).
The rationale for NLR is that it measures the tumor inflammatory response (neutrophilia) and the host immune response (lymphopenia). One of its strengths is its simplicity of calculation, defined as the number of absolute circulating neutrophils divided by the absolute lymphocyte count(18, 19, 20, 21). Neutrophils exhibit dual behavior in the tumor microenvironment. Two subtypes or phenotypes have been established: high-density neutrophils (HDN) and low-density neutrophils (LDN)(14).
The HDN subtype has antitumor activity and directly affects tumor cells or indirectly stimulates T-cell-mediated immunity. Conversely, the LDN subtype exhibits pro-tumoral activity that promotes progression, mediated by two mechanisms. LDNs promote the activation of suppressor T cells by secreting Arginine, regulating angiogenesis through the stimulation of vascular endothelial growth factors (VEGF). At the onset of inflammation, neutrophils have the HDN phenotype, but upon resolving this acute inflammation, the LDN phenotype accumulates. Chronic inflammation, as seen in cancer, results in the generation and accumulation of the LDN phenotype, leading to an unfavorable neutrophil phenotype. The proportion of LDN neutrophils increases with the tumor burden(14).
On the other hand, lymphopenia is associated with reduced host immunity and indirectly with the stimulation of suppressor T cells(20). These cells contribute to the decreased antitumor immune activity, with the most well-known being CD4+ CD25+ FOXP3+ regulatory T cells (Tregs). These cells are involved in preventing the immune response and inhibiting antitumor immune responses, thus maintaining normal balance in the body. However, Treg accumulation in cancer has been linked to poor outcomes in some cancer types(21, 22).
The importance of NLR in ALM has not been well-studied. However, there are few studies in Asia investigating its prognostic value(23, 24). Yu et al. evaluated baseline peripheral blood biomarkers to predict early-stage ALM outcomes treated with IFNα-2b and found that NLR ≥2.35 was associated with poor recurrence-free survival and OS(25). Conversely, Jung et al. conducted a retrospective study in an Asian population to evaluate the efficacy of Ipilimumab in melanoma, finding that 31% had advanced-stage ALM. They found that NLR levels greater than 5 represented an independent poor prognostic factor for survival. Elevated NLR levels were observed in patients experiencing progressive disease, and low NLR predicted longer progression-free survival (PFS) and OS(26). Additionally, Lee et al. conducted a retrospective cohort of 152 patients, of which 58 (38%) had ALM. NLR levels > 2.1 were associated with poorer progression-free survival (median 6.9 vs. 2.4 months, p = 0.015) and OS (median not reached vs. 10.4 months, p < 0.001)(27, 28). Our study aligns with these findings, confirming the prognostic role of NLR in ALM survival in the South American population.
This study has limitations. Regarding the study design, being a retrospective cohort with a small population compared to other series, the power and external validity are limited. Moreover, it was a single-center study conducted in one institution where patients were referred for surgical, adjuvant, or first-line treatment, thus requiring careful extrapolation of the study results. Additionally, data collection was based on the review of clinical records, posing a potential selection bias.
This study reports that ALM is a frequent and aggressive subtype of cutaneous melanoma in our country. This is the first Latin American report investigating the prognostic value of NLR in ALM, marking an initial step toward better understanding its tumor microenvironment. It also holds potential for targeting new therapeutic approaches in the future. Further research in prospective trials is needed.
ConclusionThe present study showed that NLR levels greater than 3.5 are associated with lower overall survival independently of other variables in patients with acral lentiginous melanoma, thus representing a notable prognostic marker in this population.
Conflict of interest Statement: The authors declare no conflicts of interest.
Authorship contributions: All authors significantly contributed to the research work.
Conceptualization, J.A.M, B.B.G, M.E.A.C; methodology, B.B.G, M.E.A.C; analysis, J.A.M, B.B.G;
investigation, M.E.A.C, J.A.M, B.B.G; data curation, J.A.M, B.B.G, M.E.A.C; writing – original
draft preparation, B.B.G, M.E.A.C; writing – review and editing, J.A.M, B.B.G, M.E.A.C;
visualization, J.A.M, B.B.G, M.E.A.C; supervision, J.A.M, B.B.G, M.E.A.C. All authors have read
and agreed to the published version of the manuscript.
Funding Sources Statement: Self-funded.
Received: July 5th, 2024
Approved: July 27th, 2024
Corresponding author: Joseph Alburqueque-Melgarejo
Address: Callao, Lima, Peru
Phone: +51-979 862 474
Email: jalburqueque@cientifica.edu.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES