ORIGINAL ARTICLE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2024 - Universidad Ricardo Palma10.25176/RFMH.v24i3.6682
EFFECT OF CONSUMPTION OF THE FRUIT EXTRACT OF “camu camu” ON THE INTEGRITY OF THE SPERM DNA OF MICE PRETREATED WITH UNIQUE DOSE OF CYCLOPHOSPHAMIDE
EFECTO DEL CONSUMO DEL EXTRACTO DEL FRUTO DE “camu camu” EN LA INTEGRIDAD DEL ADN ESPERMÁTICO DE RATONES PRETRATADOS CON DOSIS UNICA DE CICLOFOSFAMIDA
Jose Luis Pino Gaviño
 1,2,a,
Carlos Bell Cortez
1,2,a,
Carlos Bell Cortez
 3,b,
Pilar Valeria Pino Velásquez
3,b,
Pilar Valeria Pino Velásquez
 1,d,
1,d,
Jacquelyne Zarria Romero
 1,c
Nilda Oliveros Rodríguez
1,c
Nilda Oliveros Rodríguez
 4,a,
Betty Shiga Oshige
4,a,
Betty Shiga Oshige
 1,2,e
1,2,e
1 Laboratory of Reproduction and Developmental Biology, Institute of Biological Sciences Research
“Antonio Raimondi”, Faculty of Biological Sciences, Universidad Nacional Mayor de San Marcos (UNMSM). Lima –
Peru.
2 Center for Research in Natural Resources (CIRNA, by its Spanish acronym). Vice-Rectorate for
Research and Graduate Studies. UNMSM.
3 Laboratory of Analytical Chemistry, Institute of Pharmaceutical Sciences and Natural Resources
"Juan De Dios Guevara". Faculty of Pharmacy and Biochemistry. (UNMSM). Lima – Peru.
4 Laboratory of Radiobiology, Institute of Biological Sciences Research “Antonio Raimondi”,
Facultad de Ciencias Biológicas (UNMSM). Lima – Peru.
a Biologist.
b Doctor of Pharmacy and Biochemistry.
c Doctor of Biological Sciences.
d Master in Assisted Reproduction Procedures.
ABSTRACT
Introduction: Myrciaria dubia known as “camu camu” is a fruit that grows in the Amazon and its
main characteristic is its high content of vitamin C. Ascorbic acid has a protective role in
spermatogenesis as it is a compound that has excellent reducing action. The purpose of this research was
to evaluate in vivo the cytoprotective capacity of the aqueous extract of the fruit of Myrciaria dubia
(Kunth) McVaugh “camu-camu” against the mutagenic damage produced by the antineoplastic drug
cyclophosphamide (CP) on the male germ line.
Methodology: Mice (n= 60) were divided into five treatment groups: T1= negative control (without
treatment); T2 ingested the aqueous extract (10mgkg-1), T3 ingested the aqueous extract (50mgkg-1), T4
ingested the aqueous extract (100mgkg-1); T5 is the positive control. All of them were injected with a
single dose of CP (50 mgkg-1) intraperitoneally. Treatment with camu-camu continued for 45 days, then
the mice were euthanized to determine sperm quality and the frequency of DNA damage using the Index
protocol. Sperm DNA fragmentation – Halomax protocol.
Results: The effect of camu-camu extract was observed in all trials (p< 0.05) compared to the
negative control. Group T4, which was administered the highest concentration of the aqueous extract of
the fruit, evidenced the cytoprotective effect of camu-camu (p< 0.05).
Conclusion: The damaging effect on DNA due to the oxidative action of CP could be inhibited by
the aqueous extract of the “camu camu” fruit.
Keywords: Camu-camu, Myrciaria dubia, Cyclophosphamide, DNA fragmentation, mouse, semen. (source:
MeSH NLM)
RESUMEN
Introduccion: Myrciaria dubia conocido como “camu camu” es una fruta que crece en la Amazonía y
tiene como principal característica su alto contenido de vitamina C o ácido ascórbico, el cual tiene el
rol de protección en la espermatogénesis por ser un compuesto con excelente acción reductora. El
proposito de esta investigacion fue evaluar la capacidad citoprotectora in vivo del extracto acuoso del
fruto de Myrciaria dubia (Kunth) McVaugh “camu-camu” frente al daño mutagénico producido por el
antineoplásico ciclofosfamida (CP) sobre la línea germinal masculina.
Metodología: Se utilizaron ratones (n= 60) divididos en cinco grupos tratamiento: T1= control
negativo (sin tratamientos); T2 ingirió el extracto acuoso (10mgkg-1), T3 ingirió el extracto acuoso
(50mgkg-1), T4 ingirió el extracto acuoso (100mgkg-1); T5 es el control positivo (se le administró
solamente CP). A todos se inyectaron una dosis única de CP (50 mgkg-1) vía intraperitoneal., El
tratamiento con camu-camu continúo por 45 días, luego los ratones fueron eutanizados para determinar la
calidad espermática y la frecuencia del daño al ADN mediante el protocolo de índice de fragmentación de
ADN espermático – protocolo Halomax.
Resultados: Se observó en todos los ensayos el efecto del extracto de camu-camu (p< 0,05)
respecto al control. El grupo T4, el cual se administró la mayor concentración del extracto acuoso del
fruto (100 mgkg-1), evidenció el mayor efecto citoprotector del camu-camu (p< 0,05).
Conclusión: El efecto dañino al ADN por la acción oxidativa del CP podría estar siendo inhibido o
modulado por el extracto acuoso del fruto de “camu camu”.
Palabras clave: camu-camu, Myrciaria dubia, ciclofosfamida, fragmentación del ADN, ratón, semen.
(fuente: DeCS-BIREME)
INTRODUCTION
Infertility is defined by the World Health Organization as a disease of the reproductive system
characterized by the inability to achieve a clinical pregnancy after 12 months or more of regular
unprotected sexual intercourse(1). This condition affects nearly 20% of
couples of reproductive age,
with the male factor contributing to 50% of the cases. The first analysis performed on a man visiting an
assisted reproduction center to predict his fertile potential is the semen analysis, which generally
includes macroscopic and microscopic examination of the seminal fluid. Additionally, new complementary
tests are now considered, evaluating other aspects of sperm such as the integrity of their genetic
material(2).
The importance of analyzing sperm DNA lies in the fact that various studies have shown that the
integrity of sperm DNA affects clinical outcomes in assisted reproduction treatments. Despite the
information provided by the semen analysis to evaluate sperm quality, approximately 10% to 15% of men
diagnosed with infertility present semen parameters within normal ranges but may have defects in sperm
DNA. Sperm DNA strand breaks are attributed to several causes, including excessive production of free
radicals in the ejaculate, as well as exposure to environmental, occupational factors, and toxic
habits(3). High sperm DNA damage has been correlated with infertility,
defective embryonic development,
implantation failure, and an increase in recurrent miscarriages(4).
Cyclophosphamide (CP) [N, N-bis(2-chloroethyl) tetrahydro-2H-1,3,2-oxazaphosphorin-2-amino 2-oxide] is
an alkylating agent commonly used as an antineoplastic and immunosuppressive drug. CP's cytotoxicity is
mediated by DNA alkylation at the N7 position of guanine and the formation of DNA-DNA and DNA-protein
cross-links, as well as single-strand DNA breaks(5-7).
Cyclophosphamide induces infertility by
interrupting meiosis before the pachytene stage, causing genotoxic damage to the germline and impairing
Leydig cells(8-10). Eukaryotic cells, to maintain genetic
stability, halt their cell cycle, which allows
for the activation of DNA repair mechanisms(11). However, when the damage
is severe, cell death pathways
such as apoptosis are activated(12). Apoptosis describes a unique
morphological pattern of cell death
characterized by chromatin condensation, membrane blebbing, and DNA fragmentation; this mechanism plays
an important role in the homeostasis of multicellular organisms. Abnormal apoptosis function has been
associated with several human diseases, including neurodegenerative disorders and cancers.
"Camu camu" (Myrciaria dubia) is a fruit that grows in the Amazon and is notable for its high vitamin C
or ascorbic acid content(13). Ascorbic acid has been reported to have a
protective role in
spermatogenesis due to its excellent reducing action, making it a good antioxidant(14-16). Reports have
shown that the aqueous extract of Myrciaria dubia H. B. K. Mc Vaugh "camu camu" has an antimutagenic
effect against damage caused by fluoride salts, demonstrated through the in vivo micronucleus assay in
mouse bone marrow. When administered beforehand, it also has a cytoprotective effect on the same cell
line(17). Another study conducted in our laboratory confirmed the
protective effect of camu camu on
three cell lines previously treated in vivo with potassium bromate(18).
Based on the reported scientific
evidence, the objective of this research is to determine whether "camu camu" can reverse the negative
effect of CP on the male germline in mice, focusing this evaluation on the DNA fragmentation index of
sperm. The achieved results could be extrapolated for human use, thereby restoring fertility in patients
who have required the use of this drug in their cancer treatment.
The Halomax test is a technique used to identify and evaluate sperm with damaged DNA. It identifies
sperm with damaged genetic material (DNA) and differentiates them from those without damage. This test
establishes the proportion of sperm with fragmented DNA in the total analyzed sample. It is estimated
that, using normal reproduction methods, a percentage of sperm with fragmented DNA above 30% reduces or,
in some cases, eliminates the possibility of achieving a full-term pregnancy.
The purpose of this research was to evaluate in vivo the cytoprotective capacity of the aqueous extract
of Myrciaria dubia (Kunth) McVaugh "camu camu" fruit against the mutagenic damage caused by the
antineoplastic cyclophosphamide (CP) on the male germline in mice.
METHODS
DESIGN AND STUDY AREA
Preclinical experimental study in the field of experimental biology.
POPULATION AND SAMPLE
The sample consisted of 60 male albino BALB/c mice (Mus musculus) aged 6 to 8 weeks, obtained from the
animal facility of the Instituto Nacional de Salud in Lima, Peru. The treatment groups were administered
the aqueous extract via nasogastric tube No. 18 (Fisher Scientific, Pittsburgh, PA, USA). The mice were
maintained under standard animal facility conditions: 14-hour light/10-hour dark photoperiod,
temperature of 25°-27°C, relative humidity of 90%, with free access to a pellet diet (Bedoce, Peru) and
water ad libitum. After an acclimatization period in the faculty's animal facility, the mice were
randomly distributed into cages in five treatment groups (n=12). On the study's start day, they were
administered CP (50 mgKg-1) once intraperitoneally, except for the negative control group.
STUDY VARIABLES
The present study evaluated reproductive organ weights, semen analyses, motility analysis, vitality
analysis, sperm morphology analysis, precise sperm count, plasma membrane integrity, and sperm DNA
fragmentation index evaluation.
PROCEDURES:
Plants
"Camu camu" fruits, Myrciaria dubia (Kunth) McVaugh, were collected in the city of Pucallpa, Peru;
transported by air to Lima and immediately transferred to the Laboratory of Reproduction and
Developmental Biology of the Universidad Nacional Mayor de San Marcos (UNMSM). The plants were certified
by the Botany Department of UNMSM. In the laboratory, the fruit was weighed and blended; the pulp of the
"camu camu" fruits was extracted and dried at 60°C for 24 hours in a dry air convection oven.
Subsequently, a 10% (w/v) aqueous extract was prepared for 24 hours at 60°C. After 24 hours, the extract
was decanted, filtered, quantified, and stored at -20°C; another 2% (w/v) final aqueous extract was
prepared from this extract. The "camu camu" fruit was lyophilized and stored for later use. The
distribution of the lyophilized "camu camu" was resuspended in distilled water as a vehicle in three
different doses (10 mgKg-1, 50 mgKg-1, and 100 mgKg-1). "Camu camu" was administered daily via Fisher
nasogastric tube No. 18 for 45 days.
Experimental Design
The mice were separated into cages with the following distribution: a negative control group NC (n=12)
was administered saline solution intraperitoneally for the same period; a group T2 (n=12) was
administered camu camu extract (10 mgkg-1 BW) for 45 days; a group T3 (n=12) was administered camu camu
extract (50 mgkg-1 BW) for 45 days; a group T4 (n=12) was administered camu camu extract (100 mgkg-1 BW)
for 45 days; a group T5 (positive control) was administered cyclophosphamide intraperitoneally (50
mgKg-1 BW) once.
Throughout the treatments, body weights were obtained daily, and at the end, all specimens from each
group underwent two evaluations: sperm analysis and sperm DNA fragmentation index evaluation.
After treatments, the mice were euthanized and dissected to separate reproductive organs, isolate them
from fat bodies, and place them in saline solution at 37°C to perform the respective sperm analyses
according to WHO-approved parameters(19), including motility analysis,
vitality analysis, sperm
morphology analysis, precise sperm count, plasma membrane integrity, and sperm DNA fragmentation index
evaluation.
Obtaining Reproductive Organs
With the aid of a stereoscope, the following organs from the male reproductive system (right and left
sides) were separated: testis, head and body of the epididymis, tail of the epididymis, and vas
deferens. They were then weighed and maintained in saline solution at 37°C during the sperm analysis
protocol application. The epididymal tail was sectioned in 0.5 mL of phosphate-buffered saline (PBS) at
37°C for sperm DNA fragmentation analysis following the Halomax Kit protocol (HALOTECH DNA SL). Sperm
with fragmented DNA are considered those with a large halo and chromatin dispersion spots, while sperm
without fragmented DNA have a small and compact chromatin dispersion halo.
STATISTICAL ANALYSIS
The results were properly tabulated and entered into Excel 2007 software to be processed using SPSS
version 17.0 for Windows. Results will be shown as mean ± standard deviation (SD) and contrasted using
ANOVA with Levene's test (to assess homogeneity of variances), Kolmogorov-Smirnov test (normal
distribution of weights and sperm concentration), and Tukey and Bonferroni tests for parametric data
(morphology, vitality, and sperm integrity) with significance levels of p<0.05 and p<0.01.
Ethical Aspects
The care and handling of the animals were conducted in accordance with the ethical guidelines of the
Universidad Nacional Mayor de San Marcos and the National Research Council for the care and use of
laboratory animals (20).
RESULTS
No significant differences were observed in the increase of body weight, weight of the testes,
epididymis, and prostate (p>0.05) (Table I); as well as in sperm morphology (not included in the
tables), among the groups analyzed during the experiment. Similarly, the results of vitality, motility,
membrane integrity, and sperm count are detailed in Table II.
The cytological differences between fragmented and non-fragmented sperm are shown in Figures 1 and 2. In
Figure 1, representing the positive control group (cyclophosphamide only, 50 mgKg-1 BW), large halos are
observed, indicating sperm DNA damage in a value higher than those in Figure 2, where sperm from group 4
(cyclophosphamide only, 50 mgkg-1 BW) + camu camu extract (100 mgkg-1 BW) show few sperm with large
halos. It is important to note that the presence of the flagellum differentiates sperm from other
possible cells involved.
|
GROUP |
TESTICULAR WEIGHT |
EPIDIDYMAL WEIGHT |
PROSTATE WEIGHT |
|---|---|---|---|
|
Negative Control |
0,1277 ± 0,0025 |
0,0413 ± 0,0012 |
0,0513 ± 0,0022 |
|
Treatment 1 |
0,1066 ± 0,0054 |
0,0401 ± 0,0012 |
0,0510 ± 0,0016 |
|
Treatment 2 |
0,1121 ± 0,0046 |
0,0219 ± 0,0239 |
0,0500 ± 0,0008 |
|
Treatment 3 |
0,1123 ± 0,0033 |
0,0377 ± 0,0021 |
0,0459 ± 0,0017 |
|
Positive control |
0,0899 ± 0,0691 |
0,0338 ± 0,0028 |
0,0428 ± 0,0029 |
p<0,05 Treatments vs control
|
Treatment |
Motility |
Vitality (viable) |
Membrane Integrity (viable) |
Sperm count (million/ml) |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
PM |
NPM |
IM |
|||||||||||||||||||||||
|
NC |
54.399±14.311 |
11.623±8.096 |
33.978±10.749 |
60.944±20.221 |
60.342±13.745 |
1.079x106±51.563 |
|||||||||||||||||||
|
PC |
49.564±11.361 |
12.558±9.965 |
37.878±10.624 |
65.722±18.777 |
56.978±14.844 |
1.310x106±25.797 |
|||||||||||||||||||
|
10mgkg-1 |
42.121±20.103 |
12.476±9.508 |
45.403±18.788 |
46.401±31.631 |
51.555±20.636 |
1.059x106±25.658 |
|||||||||||||||||||
|
50mgkg-1 |
29.391±12.306** |
14.559±7.600 |
56.050±14.123** |
40.714±13.082 |
49.289±18.396 |
0.795x106±30.568** |
|||||||||||||||||||
|
100mgkg-1 |
46.297±7.147 |
18.446±7.692 |
35.257±5.886 |
56.787±14.220 |
58.867±9.421 |
1.646x106±29.698 |
|||||||||||||||||||
NC=negative control; PC=positive control. PM (rapid and slow progressive motility) Sperm moving
actively in a straight line or large circles regardless of speed. NPM (Non-Progressive Motility)
Sperm showing movement without locomotion.. IM (Immotility) Complete absence of motility.
Values are expressed as Mean ± SD
**Significant for p<0.05 compared to positive control.
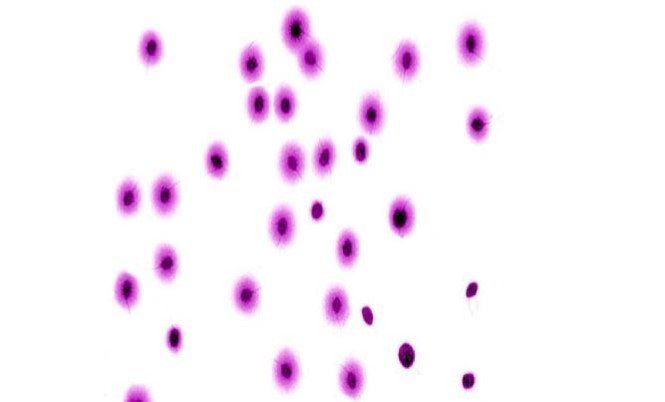
Figura 1. Resultados del Test Halomax en espermatozoides de ratón tratados con ciclofosfamida (50 mgKg-1 PC). La presencia de halos grandes, evidencia daño en el ADN, signo de fragmentación 400x
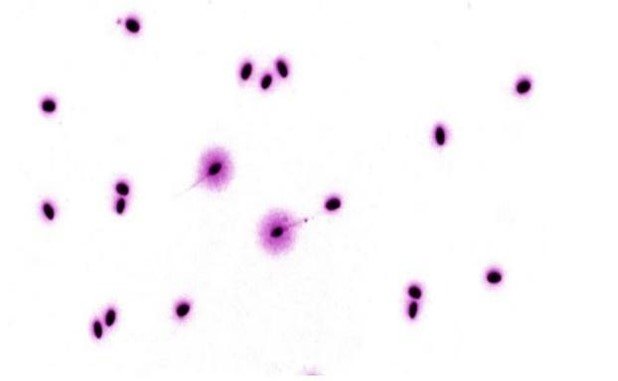
Figura 2. Resultados del Test Halomax en espermatozoides de ratón tratados con ciclofosfamida (50 mgKg-1 PC) y extracto acuoso de camu camu (100mgKg-1 PC). La presencia de opocos espermatozoides fragmentados sugiere el efecto protector del fruto. 400x
DISCUSSION
Reproduction and fertility are the foundation of species continuity. However, when referring to our own
species, this premise goes beyond simply fulfilling our biological purpose. While infertility as a
disease does not cause death, it often leads to situations that can be deemed as lacking psychological
and social well-being(1). From this perspective, any effort to generate
knowledge that helps individuals
achieve conception should be considered a priority from both biological and clinical viewpoints.
The protective effect of antioxidant substances against genotoxicity can occur in three ways: by
decreasing the assimilation of pro-oxidant genotoxicants, preventing their formation within the diet
itself; as a reducing agent at the sites of pro-oxidant action, and by inducing detoxifying enzymes
capable of reducing active oxygen intermediates(3-16).
Analyzing the protective effect of the aqueous extract of Myrciaria dubia fruit through the sperm DNA
integrity test showed no significant differences between NC and the treatment groups. This result
suggests a protective effect of the aqueous extract of camu camu fruit against CP oxidative damage.
Additionally, it was determined that oral supplementation with vitamin C in humans reduces DNA damage
induced by hydrogen peroxide (H2O2). In vivo studies in human cells and in vivo studies in rodents have
demonstrated that high intracellular concentrations of ascorbic acid reduce mutations caused by
oxidative stress from KBrO3(20). It is likely that the high content of ascorbic acid (vitamin C) in camu
camu fruit is responsible for the protective effect observed in the results, as a similar number of
grade 0 cells were found between T1 and T4.
It is known that CP induces permanent alterations due to different types of damage, which can be
detected in a micronucleus test by blocking cytokinesis(22,23). These findings indicate that CP induces
DNA damage through various mechanisms besides oxidative stress.
CONCLUSIONS
It is concluded that the oral administration of aqueous extract of camu camu can counteract, modulate,
and neutralize the effects of CP, as evidenced by the reduced rate of sperm with nuclear DNA damage in
treated samples.
LIMITATIONS OF THE STUDY
The study's limitations were logistical in nature at the institution where the work was carried out,
primarily due to unplanned suspensions of activities during weekends (sometimes starting on Friday),
which disrupted the dosing and continuous monitoring of the animals. Additionally, the inability to
access the research pavilion on Sundays due to a lack of security personnel caused delays and repetition
of the experimental design.
Authorship contribution:
The authors participated in the conception of the idea, project design, development, data collection
and interpretation, results analysis, and manuscript preparation.
Funding:
Vice-Rectorate for Research of the Universidad Nacional Mayor de San Marcos (project with monetary
funds, code 151001171)
Conflict of interest statement:
The authors declare no conflict of interest in the publication of this article.
Received:
May 12, 2024.
Approved:
July 11, 2024.
Corresponding author:
José Luis Rafael Pino Gaviño
Address:
Av. German Amézaga 375, Cercado Lima-Perú
Phone number:
992169186
Email:
jpinog@unmsm.edu.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES
