Introducción
Congenital hearing loss has a prevalence of 1 to 2 per 1,000 newborns and has negative consequences for language, speech, cognitive, and socio-emotional skills
1
. The hearing loss experienced by patients with microtia and aural atresia is usually conductive or mixed in most cases
2
.According to the World Health Organization, more than 5% of the global population suffers from disabling hearing loss and requires rehabilitation (34 million children)
3
. Microtia is a congenital defect of the auricle at birth, ranging from incomplete development to complete absence, and can occur in isolation or associated with other malformations
2
4
5
. A patient with microtia, without an associated syndrome, can generally live a normal and productive life
2
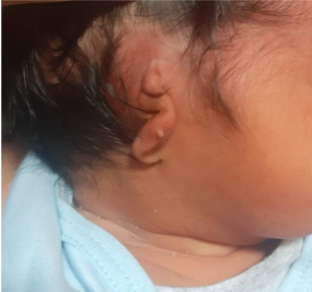
. The most common presentation of microtia is grade III, which consists of hypoplasia of the entire cartilaginous framework with a lobular remnant (see figure 1)
6
. (véase figura 1)
Methodology for search and selection of results
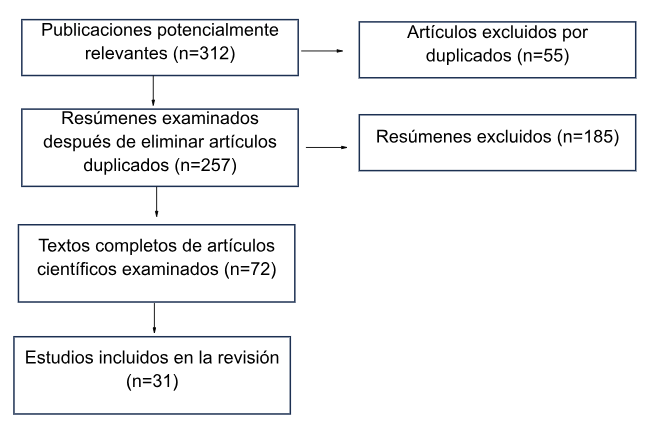
The review was conducted through an electronic bibliographic search based on evidence, with articles in English and Spanish from PubMed/Medline, Scopus, Clinical Key, and SciELO, covering the period from 2010 to 2023 (see figure 2). All references in both Spanish and English with the keywords "congenital microtia" and "congenital aural atresia" were searched. Review and research articles were evaluated, generally published within the last five years. Once relevant articles were identified, the inclusion criteria were as follows:
1. Articles examining the diagnosis and treatment of congenital microtia and aural atresia, and 2. Articles addressing the topic with a systematic methodology (quantitative, qualitative, or other approaches)
After a full-text evaluation, 31 studies were selected for inclusion in this article. The review process spanned four months (from February 2023 to June 2023). This work is part of the Research Lines of the Universidad Ricardo Palma 2021-2025
31
.
Figure 2:
Flowchart of article search and information selection
Development of the topic
Epidemiology
Microtia has an incidence of 1 to 10 per 10,000 births
6
12
, with significant social implications as affected individuals may experience psychological problems due to this visible and difficult-to-conceal defect
13
. Microtia is associated with aural atresia in 75% of cases
9
. In Mexico, a prevalence of 7.37 per 10,000 live births has been reported
14
. In Peru, there is no national data to determine the prevalence of microtia and aural atresia
15
.
Microtia can be unilateral or bilateral
29
. 90% of microtia cases are unilateral, with the right ear being more affected than the left ear
5
6
11
12
16
and it is more common in males than females
5
7
12
. This malformation is associated with a syndrome in 30-60% of patients
6
17
.
In unilateral cases, microtia is defined as a size discrepancy between the ears that exceeds the normal variation
18
. Bilateral microtia is defined as an external ear length more than two standard deviations below the mean. In severe cases, the auricle is completely absent (anotia).
Embryology and anatomy
The somatic ectoderm plays a role in the formation of the external and internal ear, forming the epithelial elements of the auricle, external auditory canal, the outer layer of the tympanic membrane, and the membranous labyrinth of the inner ear
19
.
The auricle develops from three auricular prominences of the first branchial arch
13
20
. It forms from the fusion of six mesenchymal buds or His auricular prominences on the surface of the embryo during the fifth week of intrauterine development, with its development completing by the twelfth week
21
.
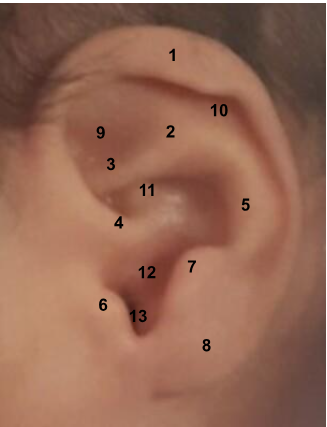
The anatomy of the auricle is shown in Figure 3.
Figure 3:
Anatomy of the auricle. 1. Helix, 2. Superior crus, 3. Inferior crus, 4. Helix root, 5. Antihelix, 6. Tragus, 7. Antitragus, 8. Earlobe, 9. Triangular fossa, 10. Scaphoid fossa, 11. Concha cymba, 12. Concha cavum, 13. Intertragal notch.
Source: Instituto Nacional Materno Perinatal (INMP). (INMP).
The external auditory canal forms from the 8th week, and its epithelium develops from the first branchial groove
22
. It measures 2.5 cm, extending from the concha to the tympanic membrane and consists of two zones:
- 1. Outer cartilaginous third
- 2. Inner two-thirds bony portion
22
It has an S-shape, initially directed inward, forward, and slightly upward; then it curves backward and upward, and finally inward, forward, and slightly downward
22
. Early disruptions in this process lead to anotia or microtia, while later disruptions result in minor auricular malformations.
Congenital anomalies of the external and middle ear affect structures derived mainly from the first and second branchial arches, the first groove, and the first pharyngeal pouch
Etiopathogenesis
The causes are not fully understood and remain unclear
17
23
. Genetic and environmental factors can result in malformation during the embryogenesis of the external, middle, or inner ear, primarily between the third and tenth weeks of gestation
19
. Embryologically, there is a failure in the development and/or migration of mesodermal and cartilaginous components
23
.
External auditory canal atresia may be due to the failure of reabsorption of the meatal plug or overdevelopment of the Reichert’s cartilage (second branchial arch)
19
. Malformations of the malleus and incus may result from defective differentiation of Meckel’s cartilage (first branchial arch), leading to malformed ossicles or abnormal fixation of the malleus and incus
23
.
Associated factors
Ethnicity, male gender, low birth weight, acute maternal viral illness, maternal education level, maternal diabetes, multiple births, and maternal use of thalidomide, retinoids, aminoglycosides, alcohol, and smoking during pregnancy are associated factors for microtia
9
23
24
29
23
. Additionally, infections like rubella, cytomegalovirus, or toxoplasma gondii, and metabolic disorders such as hypothyroidism or endemic cretinism are associated with microtia
23
24
. Higher folate intake during pregnancy has been found to reduce the incidence of microtia
24
. Less than 50% of patients with microtia are associated with syndromes such as craniofacial microsomia, Treacher Collins syndrome, Goldenhar syndrome, Crouzon syndrome, Moebius syndrome, Fanconi syndrome, DiGeorge syndrome, Pierre Robin syndrome, CHARGE syndrome, VACTERL syndrome, labyrinthine aplasia, and branchiootorenal syndrome
7
9
23
24
. Additionally, a study has associated microtia with altitude (>2000 meters above sea level) in certain cities.
28
.
Classifications
Tanzer
27
and Meurman
25
classified microtia (see Tables 1 and 2).
Table 1. Tanzer Classification
| Grade |
Description |
| I |
Anotia (see figure 3) |
| II |
Complete hypoplasia (microtia):
A. With external auditory canal atresia
B. Without external auditory canal atresia
|
| III |
Hypoplasia of the middle 1/3 of the auricle |
| IV |
Hypoplasia of the upper 1/3:
A. Horn-shaped or cup-shaped ear (see Figure 4)
B. Cryptotia
|
Tabla 2. Meurman Classification.
| Grade |
Description |
| I |
Small auricle, but harmonious |
| II |
Vestigial remnants of the auricle, external auditory canal atresia |
| III |
Almost complete absence of the auricle, remnant in the form of a lobe |
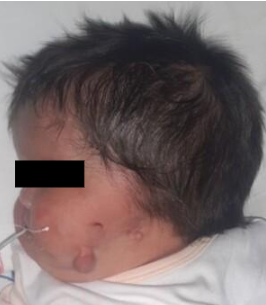
Figure 4
Patient with anotia in the left ear plus melotia
Source: Instituto Nacional Materno Perinatal.
Figure 5
Patient with hypoplasia of the upper third of the right ear auricle
Source: Instituto Nacional Materno Perinatal
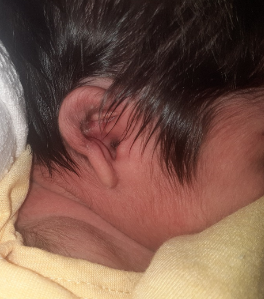
Diagnosis
The diagnosis of microtia and aural atresia is clinical, supported by complementary exams. During the clinical exam, the shape of the auricle (smaller than normal ears), its implantation, and stigmata (fistulas, appendages, or nodules) should be observed
23
29
The meatus, external auditory canal (abnormally narrow, blocked, or absent), and the tympanic membrane should also be examined
23
29
. Additionally, it is important to assess the temporomandibular joint (soft tissue dysplasia) and the ascending branch of the mandible, as well as the appearance and conformation of the cranial sutures. Facial asymmetries, maxillary hypoplasias (upper or lower), oral opening, cleft palate, or submucosal cleft, and characteristics of the neck, chest, and upper and lower limbs should also be evaluated, along with the presence of branchial cysts
23
.
Complementary examinations
Otoacoustic emissions are recommended to evaluate the healthy ear. A microphone in the external auditory canal detects these low-intensity otoacoustic emissions
1
.
The pediatrician or family physician usually has the first contact with the child and must be aware of the risk factors for hearing loss and the need for hearing screening. The otolaryngologist must have the necessary equipment and training in pediatric diagnosis.
Auditory evoked potentials are the complementary exams of choice. Brainstem auditory evoked potentials (BAEP) or BERA
1
measure the electrical response of the auditory pathway at the brainstem level, including the cochlea and retrocochlear pathway, using surface electrodes. Additionally, auditory steady-state response (ASSR) measures the hearing level and can evaluate the bone conduction pathway.
Free-field audiometry, play audiometry, and tonal audiometry are indicated depending on the child’s age
21
25
. Tympanometry is recommended for permeable canals or the ear contralateral to dysgenesis to determine possible malformations of the ossicular chain in apparently normal ears
23
.
Regarding imaging studies, a computed tomography (CT) scan of the temporal bones with thin axial and coronal slices without contrast is requested. This allows for the evaluation of the temporal bone, tympanic bone, mastoid, middle ear cavity, its relationship to the facial nerve, ossicular chain, and the conformation of the bony labyrinth. A CT scan of the temporal bones is performed around the ages of five to six years, or earlier in cases of bilateral dysgenesis or suspected cholesteatoma
25
.
Magnetic resonance imaging (MRI), specifically of the posterior fossa, is requested to evaluate the membranous structures of the cochlea, posterior labyrinth, and cranial nerves.
Treatment
A bone vibrator device, similar to a headband or soft band, is recommended before patients become candidates for osteo-integrated bone vibrator surgery to stimulate the auditory nerve
34
. The use of bone conduction devices in young patients aids in the acquisition of language skills during critical periods of life
34
.
. Reconstructive options for microtia are as follows: (see Figure 5)
Figure 6
Treatment of microtia and aural atresia.
Source: Bly RA, Bhrany AD, Murakami CS, Sie KC. Microtia Reconstruction. Facial Plast Surg Clin North Am. 2016;24(4):577-591. doi: 10.1016/j.fsc.2016.06.011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5950715/
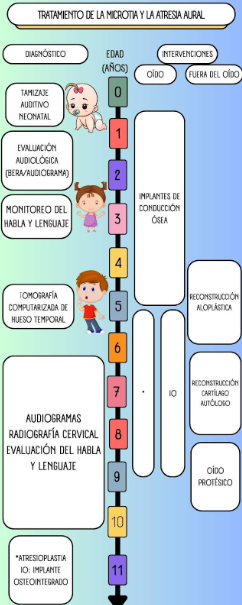
1. A portion of autologous costal cartilage placed subcutaneously.
2. Implanted artificial material, including a porous polyethylene implant placed subcutaneously or under a vascularized fascial flap and skin graft.
3. An ear prosthesis adhered to the skin with medical adhesive or through osseointegrated implants.
The management of aural atresia includes bone conduction implants, middle ear implants, and surgical reconstruction
5
,
8
.
Among these options, canaloplasty has the advantage of reconstructing the external auditory canal and reducing the need for hearing devices
8
.
As for bone conduction implants, there are BAHA (Bone Anchored Hearing Aid), Bonebridge, and Sophono.
The indications for BAHA are as follows:
Patients over five years of age with unilateral or bilateral auditory dysgenesis who present conductive or mixed hearing loss with bone conduction above 45 dB, who cannot use an air-conduction hearing aid, may be candidates for it.
Prosthetics should be considered from 3-4 months of age if the hearing loss is bilateral. When the external auditory canal is permeable on at least one side, an air-conduction prosthesis is recommended
19
.
The Bonebridge uses a bone conduction system that stimulates the cochlea directly through skull vibration
19
.
The internal components include a receiver coil attached to the floating mass transducer for bone conduction
20
.
The indications are the same as for the BAHA® device
19
and these are as follows:
In the case of unilateral microtia, most authors agree not to recommend surgical hearing rehabilitation due to surgical risks (labyrinthitis, facial paralysis, canal stenosis) and inconsistent results (insufficient hearing in at least 66% of cases).
Functional surgery is indicated when microtia is bilateral starting from the age of five. Otherwise, an osseointegrated prosthesis may be proposed
25
.
In aural atresia, surgical correction is often not the preferred treatment; the hearing outcome is not better than that of bone conduction devices, and surgery may be associated with recurrence or complications such as meatal stenosis
31
.
With the Sophono implant, the processor can be attached as soon as the surgical wound has fully healed, usually within three or four weeks. The implant remains completely hidden under the skin, causing less aesthetic disruption and reducing the risk of implant damage from manipulation
33
.
Patients should be diagnosed and treated by a multidisciplinary team: family physician, pediatrician, geneticist, pediatric audiologist, pediatric otolaryngologist, or pediatric plastic surgeon. The options for both auditory and auricular reconstruction should be considered and coordinated during the neonatal period
26
,
30
.
Conclusions
Systematic studies are needed in Latin America to determine the prevalence of congenital microtia and aural atresia. Auditory evoked potentials and audiometry are the tests of choice for cases of congenital microtia and aural atresia. Surgical correction is often not the preferred treatment, because hearing outcomes are not better than those of bone conduction devices. Additionally, the functional aspect should be prioritized over the aesthetic, as early hearing loss affects the child's language development.
Additional Information
Funding: Self-funded.
Conflict of interest statement: The author declares no conflicts of interest.
Authorship contribution DMM participated in the conceptualization, research, methodology, resources, and drafting of the original manuscript.
Author Correspondence Data
Correspondence author: Diego Marin Marín.
Address: Jr. Rio Amazonas 3215 Urb. Canto Rey. San Juan de Lurigancho, Lima, Peru.
E-mail: diego.franco.marin@gmail.com
Artículo publicado por la Revista de la Facultad de Medicina Humana de la
Universidad Ricardo Palma. Es un artículo de acceso abierto, distribuido
bajo los términos de la Licencia Creative Commons: Creative Commons
Attribution 4.0 International, CC BY 4.0
, que permite el uso no
comercial, distribución y reproducción en cualquier medio, siempre que la
obra original sea debidamente citada. Para uso comercial, por favor póngase
en contacto con revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES
4
Al-Sulaimani AK, Al-Khabori MS, Haridi KM, Al-Busaidi SS.
Prevalence and characteristics of microtia in Oman: 37 Years analysis. J Plast Reconstr Aesthet Surg. 2023;76:292-94.
doi: 10.1016/j.bjps.2022.10.047
5
Cywka KB, Król B, Skarżyński PH.
Effectiveness of Bone Conduction Hearing Aids in Young Children with Congenital Aural Atresia and Microtia. Med Sci Monit. 2021;27:1-8.
doi: 10.12659/MSM.933915
6
Truong MT, Liu YC, Kohn J, Chinnadurai S, Zopf DA, Tribble M, et al.
Integrated microtia and aural atresia management. Front Surg. 2022;9:1-18.
doi: 10.3389/fsurg.2022.944223
8
Kim MB, Cho YS.
Acoustic Reflex After Surgical Repair in Patients with Congenital Aural Atresia. J Int Adv Otol. 2022;18(6):482-87.
doi: 10.5152/iao.2022.21514
9
Abrol A, Bly R, Sie KCY, Bhrany AD.
Contemporary Management of Microtia. Facial Plast Surg. 2022;38(4):393-404.
doi: 10.1055/a-1854-2352
10
Volgger V, Schießler IT, Müller J, Schrötzlmair F, Pollotzek M, Hempel JM.
Audiological results and subjective benefit of an active transcutaneous bone-conduction device in patients with congenital aural atresia. Eur Arch Otorhinolaryngol. 2022;279(5):2345-52.
doi: 10.1007/s00405-021-06938-8
11
Yang L, Chen P, Liu Y, Yang J, Zhao S.
Clinical manifestations and treatment strategies for congenital aural atresia with temporomandibular joint retroposition: a retrospective study of 30 patients. J Otolaryngol Head Neck Surg. 2023;52(1):24.
doi: 10.1186/s40463-022-00615-4
12
Gautam R, Kumar J, Pradhan GS, Meher R, Arya S.
Congenital Aural Atresia: What the Radiologist Needs to Know? Curr Probl Diagn Radiol. 2022;51(4):599-616.
doi: 10.1067/j.cpradiol.2021.06.017
16
Hunter A, Frias JL, Gillessen-Kaesbach G, Hughes H, Jones KL, Wilson L.
Elements of morphology: standard terminology for the ear. Am J Med Genet A. 2009;149(1):40-60.
doi: 10.1002/ajmg.a.32599
17
Shibazaki R, Satoru N.
Preferential Associated Malformation in Patients With Anotia and Microtia. The journal of craniofacial surgery. 2019; 30(1): 66-70.
doi: 10.1097/scs.0000000000004915
18
Ferrario VF, Sforza C, Ciusa V, Serrao G, Tartaglia GM.
Morphometry of the normal human ear: a cross-sectional study from adolescence to mid-adulthood. J Craniofac Genet Dev Biol. 1999 [citado el 10 de junio 2023];19(4):226-33.
Disponible en: https://pubmed.ncbi.nlm.nih.gov/10731092/
19
Orfila D, Tiberti L.
Atresia congénita del oído y su manejo. Rev. Med. Clin. Condes. 2016; 27(6): 880-91.
doi: 10.1016/j.rmclc.2016.09.018
22
Marin C, López A, Zarante I.
Microtia: una malformación olvidada. Etiología genética y estado del arte. Universitas Médica [Internet]. 2006 [citado el 05 de junio de 2023]: 47(1): 80-90. Disponible en: https://www.redalyc.org/pdf/2310/231018678008.pdf
23
Ministerio de salud.
Guía de Práctica Clínica de Microtia de la Sub Unidad de Atención Integral Especializada del Paciente de Especialidades Quirúrgicas: Guía de Práctica Clínica de Microtia [Internet]. Perú: Instituto Nacional de Salud del Niño-San Borja; 2018 [citado el 05 de junio de 2023]. 24p. Disponible en: https://www.insnsb.gob.pe/guias-de-practica-clinicas/
24
Bly RA, Bhrany AD, Murakami CS, Sie KC.
Microtia Reconstruction. Facial Plast Surg Clin North Am. 2016;24(4):577-91. doi: 10.1016/j.fsc.2016.06.011
25
Teissier N, Benchaa T, Elmaleh M, Van Den Abbeele T.
Malformaciones congénitas del oído externo y del oído medio. EMC-Otorrinolaringología. 2008;37(4):1-11. doi: 10.1016/S1632-3475(08)53552-1
26
Zhang TY, Bulstrode N, Chang KW, Cho YS, Frenzel H, Jiang D, et al.
International Consensus Recommendations on Microtia, Aural Atresia and Functional Ear Reconstruction. J Int Adv Otol. 2019;15(2):204-8. doi: 10.5152/iao.2019.7383
28
Gonzales GF.
Impacto de la altura en el embarazo y en el producto de la gestación. Rev Peru Med Exp Salud Publica. 2012;29(2):242-9. doi: 10.1590/s1726-46342012000200013
29
Chen X, Zhang R.
Microtia epigenetics: An overview of review and new viewpoint. Medicine (Baltimore). 2019;98(41):1-7. doi: 10.1097/MD.0000000000017468
30
Lancer H, Hood K, Halliday E, Tzifa K, Lloyd M, McDermott AL.
Experience of the 'Ear Glove' in children with microtia. Int J Pediatr Otorhinolaryngol. 2022;160:111254. doi: 10.1016/j.ijporl.2022.111254
31
Lee M, Cho Y, Han G, Oh J.
Current Treatments for Congenital Aural Atresia. J Audiol Otol. 2020;24(4):161-66. doi: 10.7874/jao.2020.00325
33
Escorihuela V, Llópez I, Pitarch I, Latorre E, Marco J.
Experiencia inicial con el implante osteointegrado Alpha 1 de Sophono. Acta Otorrinolaringol Esp. 2021;65(6):361-64. doi: 10.1016/j.otorri.2014.01.005
34
Sun L, Ping L, Fan X, Wang J, Chen X.
Simulator Verification Is Potentially Beneficial for the Fitting of Softband Bone Conduction Hearing Devices in Young Children. Otol Neurotol. 2024;45(7):500-8. doi: 10.1097/MAO.0000000000004245