Introduction
Securing access to quality healthcare services, particularly for essential screening tests like mammograms, presents a significant challenge in developing nations. Women often encounter extensive waiting periods, sometimes extending for several meses, to undergo a mammogram. This crucial test plays a pivotal role in the early detection of breast cancer, where early diagnosis is crucial for effective treatment and enhanced survival
1
. Delays in diagnosis can significantly impact the survival of patients and their well-being, underscoring the importance of early detection. Compounding these challenges is the scarcity of resources and healthcare professionals, which hinders swift and efficient access to preventive care
2
. Such constraints underscore the pressing need for improvements in the interpretation of radiological studies and a reduction in the workload of imaging specialists. These improvements would not only optimize interdisciplinary collaboration, but also enhance patient care, particularly for critical screenings like mammograms
3
.
Technological advancements, particularly in artificial intelligence (AI), have significantly influenced numerous sectors, including healthcare
4
. Yet, in areas such as Latin America, where the healthcare infrastructure and resources may not fully support the integration of advanced diagnostic techniques based on informatic tools, the potential of these innovations seems to be underutilized. A promising application of technology is in computer-aided diagnosis (CAD) for breast cancer screening with mammograms. CAD leverages vast datasets and pattern recognition algorithms to detect anomalies within mammograms, potentially facilitating the early identification of lesions
5
. Given that breast cancer ranks among the most prevalent diseases affecting women globally, early detection is vital for enhancing patient survival rates and quality of life. Given these challenges, our study aims to examine the latest developments in artificial intelligence technology as a supplementary tool in CAD strategies for mammograms. Our goal is to foster better interdisciplinary collaboration among clinicians, radiologists, and pathologists through a state of the art (SOTA) review of the topic. By doing so, we anticipate streamlining the diagnostic workflow and elevating the efficiency of breast cancer detection and treatment processes.
1.1. Breast cancer screening
The prevalence of breast cancer among women globally is a pressing concern, with it being the most commonly diagnosed cancer and ranking fifth in terms of mortality rates in developing nations
5
. This underscores the urgent need for advancements in diagnostic methodologies to effectively and consistently identify the early signs of this disease. Early detection is essential in reducing mortality rates and catching the disease in its incipient stages, thereby broadening the options for optimal patient treatment and care
2
. Consequently, efforts to enhance diagnostic methods should aim not only to enhance accuracy, but also to ensure widespread availability of consistent mammogram interpretations. Several developed countries have initiated additional supplementary mammographic screening initiatives to analyze the effectiveness of early screening. In 2015, an assessment conducted by the International Agency for Research on Cancer analyzed data from 40 aggregated studies in different high-income regions of Europe, Australia, and North America, where such screening programs were implemented. The assessment findings indicated that the implementation of mammographic screening programs resulted in a 23% decrease in breast cancer mortality rates
5
.
In light of this challenge, AI and CAD systems hold promise in revolutionizing the breast cancer screening process. These technologies offer substantial advantages by potentially reducing the time required for radiologists to interpret images, thereby supporting healthcare professionals in their diagnostic responsibilities
6
. Through the integration of AI and CAD systems into these screening protocols, the efficiency and accuracy of breast cancer detection could be significantly enhanced, leading to improved patient outcomes.
1.2 BIRADS in mammograms
To create a line of communication through which both radiologists and other physicians with different clinical specialties can understand each other when talking about the description of mammographic findings, the Breast Imaging Reporting and Data System (BI-RADS) lexicon was developed by the American College of Radiology. The descriptors in BI-RADS have the purpose of classifying the observed masses in a mammography by the radiologist, as shown in (table 1). According to the BI-RADS classification given, it will be given a distinct clinical path of action.
Each one of the categories that findings, will point towards the nature of the lesion or the clinical interpretation. Category 1 and 2 require annual screening, and category 3 a six month follow-up test. Given the large number of masses, which can follow under these categories, the need for a more effective way to evaluate images arises. There have been instances where the variability of observation between radiologists has led to a conflict of opinions in the evaluation of an image and the lesion observed, and this discrepancy could lead to an incorrect classification and skewing of the correct steps of vigilance to be taken
5
. In an effort to improve the consistency and accuracy of mammographic interpretation, CAD systems have been developed. CAD systems use algorithms to assist radiologists in detecting and classifying abnormalities in mammograms. These systems can help reduce variability among radiologists and improve the overall quality of mammographic interpretations.
1.3 Computer Aided Diagnosis
Historically, traditional CAD systems have heavily relied on human input, employing predefined parameters to detect anomalies when used in the context of medical images, particularly concerning breast cancer diagnosis. For microcalcifications, these systems analyze high-intensity pixels resembling rods, whereas for masses, they scrutinize specific attributes, such as shapes, textures, gradients, and grayscale levels, as shown in figure 1. CAD systems are categorized into two distinct groups: detection systems, responsible for identifying potential abnormalities, and diagnostic systems, which evaluate the likelihood of disease based on abnormality features
5
. However, radiologists retain the final authority in concrete diagnosis and patient management decisions.
While traditional CAD systems have achieved comparable performance to human readers in mass detection
3
, they are limited by this need for manually crafted features. In a notable development
6
, successfully implemented a CAD system based on a Convolutional Neural Network (CNN) to classify benign and malignant breast masses. Their model utilized a combination of low and high-level deep features from different CNN layers, achieving a classification accuracy of 96.7%.
Another CAD system was proposed for the detection and classification of breast masses, utilizing a regional CNN architecture known as You Only Look Once (YOLO)
7
. YOLO is distinguished by its ability to concurrently learn Regions of Interest (ROIs) and their backgrounds. The YOLO-based CAD system is structured around four primary stages: a) preprocessing of mammogram images. b) feature extraction via multi-convolutional deep layers. c) mass detection employing a confidence model. d) breast mass classification facilitated by a fully connected neural network (FC-NN). This innovative methodology aims to bolster the efficiency and precision of breast cancer diagnosis by mitigating certain constraints inherent in conventional CAD systems. Successful application of the YOLO-based CAD system has proven to analyze mammograms with an overall accuracy of 99.7%. As mentioned previously, YOLO generates the confidence probability for each potential ROI that represents the mass position.
1.4 AI and CAD in breast cancer screening
AI and CAD are revolutionizing breast cancer screening by enhancing the accuracy and efficiency of detection. AI algorithms can analyze mammograms, identifying subtle patterns and anomalies that may indicate early stages of cancer, even before they are noticeable to the human eye. CAD systems work in tandem with radiologists, flagging areas of concern for further evaluation. This technology not only improves detection rates but also reduces the chances of false positives, leading to more effective and timely interventions. AI and CAD are transforming the landscape of breast cancer screening, offering a powerful tool in the fight against this disease.
Diagnostic accuracy of mammography is dependent on factors such as breast anatomy, tissue density, and mainly the radiologists' skill and experience. In single-readings (SR), it is estimated that 70% of missed breast cancers (BC) are due to misinterpretation, whereas 30% are attributed to lesions being overlooked. To improve the sensitivity of mammographic screenings, CAD systems have been introduced to highlight suspicious areas, including microcalcifications and masses. The U.S. Food and Drug Administration approved the first CAD system for mammography in 1998. By 2016, the utilization of digital mammography technology had risen up to 91.8% in the USA
1
.
1.5 Clinical application
The concept of CAD was first introduced by Winsberg in 1967. In the diagnostic process, CAD employs pattern recognition software to identify unfamiliar forms in images for consideration by physicians. Various imaging modalities, including mammography (MM), ultrasonography (USG), computerized tomography (CT), magnetic resonance imaging (MRI), and biopsy, are all utilized by CAD systems for breast cancer diagnosis. CAD shows promise to improve the efficiency and routine of radiologists by saving time between readings and maintaining consistency in lesion recognition. Machine learning is integrated into breast imaging through CAD, serving as a "second pair of eyes" and aiding in the interpretation and processing of accurate medical images. The primary goal of CAD is to reduce human error in observations and minimize false reports during image readings.
CAD has emerged as a major research focus in medical imaging and diagnostic radiology. In this paradigm, radiologists utilize computer-generated outputs as a "second opinion" in making final diagnostic decisions. CAD is a concept that acknowledges the equal roles of physicians and computers, distinct from automated computer diagnosis relying solely on algorithms. The aim is not for computers to outperform physicians, but for their performance to complement that of physicians. CAD systems have found extensive application in assisting physicians, particularly in the early detection of breast cancers through mammograms.
While the current performance of CAD systems is promising, it is not yet sufficient for them to function as standalone clinical detection and diagnosis systems. Various CAD systems designed to aid in the early detection of breast cancer typically progress through three stages: tumor detection, segmentation, and classification based on tumor shape and subtypes, utilizing deep learning models. Initial detection involves identifying the ROI using a faster CNN detector. CAD algorithms heavily depend on mammograms, and efforts have been made to establish breast cancer recognition and grouping images to enhance precision and overcome operator dependence. Despite the significant developments in CAD since the early computer era, challenges persist, in areas such as the algorithmic limitations, assembly of input data, preprocessing, processing, and system assessments.
2. Methodology
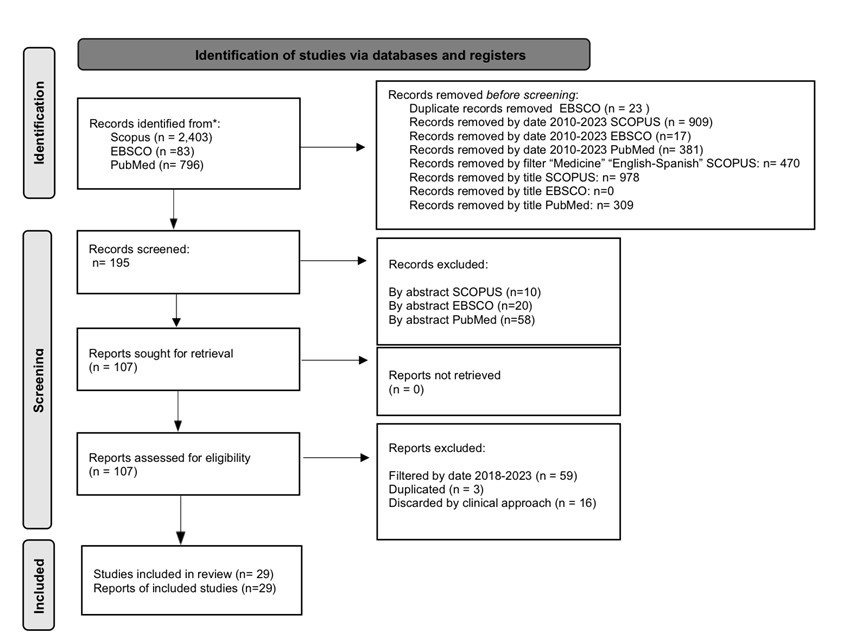
The PRISMA methodology
8
was used, following the recommendations for reviews of literature. Diagram 1 reflects the process of the search stage for this review and includes the search queries used for each database, as shown in table 2.
3. Results
In figure 2, we present an overview of the general concepts found in this work, how they can be orderly understood privileging clinical practice and pertinence. The following sections expand on each one of the elements presented in the figure.
3.1 Clinical methods and performance of CAD
Different methods such as CNN, YOLO-based CAD systems, Full Resolution Convolutional network (FrCN), and traditional CAD systems have been employed. Various datasets including digital database for screening mammography (DDSM), INbreast, Breast Ultrasound Images Dataset (BUSI), Curated Breast Imaging Subset of Digital Database for Screening Mammography (CBIS-DDSM), and private datasets have been used for the studies.
In table 3, it is presented the performance of CAD systems in identifying breast lesions based on BI-RADS categories across different papers. Metrics reported for each algorithm are sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy. Results demonstrate the effectiveness of CAD systems in assisting with detection of breast lesions across various BI-RADS categories.
From a clinical perspective, the papers analyzed offer valuable insights into the advancement of CAD systems for breast cancer detection. A study by Assari et al., in 2022
9
, focused on solid breast mass classification, employing separate models for each imaging modality and integrating them into a single Bimodal Model (BCNN). Their achievement of 90.38% accuracy and an area under the curve (AUC) of 95.82% signifies promising progress in accurately classifying breast masses, which is pivotal for effective diagnosis and treatment planning.
Similarly, in the study by Boumaraf, S. et al., in 2020
5
, mammographic masses were classified into four assessment categories using an average ensemble of models with an XGBoost classifier. Their comprehensive evaluation encompassing accuracy, AUC, sensitivity, specificity, F1 score, and Matthews Correlation Coefficient (MCC) across different datasets shows the robustness of their approach. This use of separate models for different imaging modalities and subsequent integration into a single model highlights the potential for multimodal approaches to enhance diagnostic accuracy. By leveraging these multiple assessment metrics, their study provides a deep understanding of CAD system performance, essential for its potential future clinical decision-making. Scientifically, both studies contribute to advancing CAD methodologies for breast cancer detection. Integration of diverse imaging modalities enables a more comprehensive assessment of breast masses, potentially reducing false-positive and false-negative diagnoses.
3.2 Advancements and hurdles in the global application of CAD
Recent advancements in CAD systems for breast cancer detection via mammograms are shown in table 4, in which it highlights the importance of screening mammography for early detection of breast cancer, as well as the challenges associated with CAD systems.
Further testing of different CAD models highlights the current status and challenges that they still have in breast cancer detection. While the reported performance of CAD systems is encouraging, with an average area under the ROC curve of 0.86
10
, they are not yet deemed reliable enough for standalone global clinical use. One of the primary challenges facing CAD systems is the low contrast between normal and malignant breast tissues, particularly prominent in dense breast tissue. As discussed previously, this poses a significant obstacle to accurate lesion detection and classification.
Moreover, the reliability of CAD systems is limited by their dependence on specific datasets for training and tuning, making it challenging to generalize excellent performance results reported in the literature. Addressing the variability in breast densities presents a significant challenge in the area of mammographic analysis. Failure to appropriately calibrate detection models to the specific density of the mammogram can lead to erroneous measurements and diagnostic inaccuracies. This concern underscores the critical need for leveraging a diverse array of AI tool models, meticulously trained on an extensive library of mammographic variations.
Transfer learning stands out as a pivotal strategy in this context, allowing for the optimization of model performance even in scenarios with limited data availability. Notably
6
, undertook the segmentation and classification of benign and malignant lesions, employing transfer learning methodologies with pre-trained models. Utilizing this information obtained from pre-existing models, there is the opportunity to improve diagnostic accuracy and address the variability in diagnosis associated with heterogeneous breast tissue densities.
Without substantial improvements, particularly through further advancing deep learning techniques and enhanced computational power, CAD systems are relegated to secondary opinion tools within clinical practice. These challenges underscore the pressing need for further research and technological advancements to enhance the reliability and effectiveness of CAD systems for breast cancer detection.
3.3 Clinical applications of CAD systems
An important area where the implementation of CAD would be most beneficial is in reducing the time required for radiologists to evaluate mammograms. For example, senior radiologists with extensive experience who can identify lesions more quickly than their less experienced counterparts, CAD systems can significantly enhance their efficiency. The CAD model provides a rapid analysis, allowing the experienced radiologist to swiftly verify the observation, substantially decreasing the time needed for each mammogram evaluation
11
.
However, we must ensure that, even with the faster evaluation of mammograms, the accuracy of diagnoses remains as high as possible to ensure a proper approach to the lesion. Various computational models have demonstrated higher specificity and accuracy compared to human readers using BI-RADS descriptors
12
, highlighting the potential of CAD-CESM (Computer-aided detection-contrast enhanced spectral mammography) systems to enhance breast cancer detection specificity. Although the model's sensitivity was lower than that of human readers, future work involving margin assessments demonstrates how sensitivity of CAD-CESM can improve, thereby maintaining high diagnostic accuracy.
A comprehensive overview of the diagnostic performance of CAD systems in different imaging modalities for breast cancer is provided in table 5. It includes data from multiple studies, presenting key metrics such as accuracy, sensitivity, specificity, PPV, NPV, and AUC.
The studies compare the performance of CAD systems with that of experienced radiologists across different levels of expertise. Additionally, it highlights the efficacy of CAD in detecting malignant lesions, aiding in risk assessment and differentiating between benign and malignant breast lesions. These findings are crucial for understanding the potential of CAD as a supportive tool in mammography for opportune breast cancer detection.
Overall, the results from various studies evaluating CAD systems for breast cancer detection showed significant variation in accuracy, sensitivity, specificity, PPV, NPV, and AUC. While some systems demonstrated high accuracy and detection rates, others exhibited lower sensitivity and specificity. These varying results can be attributed to the use of different CAD models, datasets, and evaluation metrics across studies. The variation in these parameters significantly influences the reported outcomes, highlighting once again the challenge in the path toward future global application of CAD systems.
3.4 Integration of AI tools
AI tools have shown promise in breast imaging, particularly in digital mammography and digital breast tomosynthesis, offering a stand-alone method for diagnosis and potentially replacing the need for a second reader
3
. Integrating AI systems into routine clinical practice could help radiologists achieve performance benchmarks and improve breast cancer screening
13
. However, developing methods for radiologists to interpret AI decisions will be crucial for efficient radiology practices and better patient care
14
.
The appearance of mammographic lesions can be linked to specific histological information, pointing us towards an estimation of breast cancer stage and risk
15
. Advances in technologies such as data analysis of high throughput radiomics features and AI-based deep transfer learning have contributed to the development of numerous CAD schemes, but further research is needed in the area of multimodal AI analysis
4
.
One study evaluated an AI CAD system that successfully marked malignancy in digital mammography, particularly detecting invasive lobular carcinoma (ILC) with high sensitivity for specific types of masses and calcifications
16
.
While some articles did not provide specific sensitivity or specificity data (table 6), the overall trend indicates a significant potential for AI in improving breast cancer diagnosis and patient outcomes, highlighting the need for further research and development in this field.
3.5 AI tools use in breast cancer assessment
In table 7, we can observe two studies conducted by Lee S et al.
17
to evaluate the agreement between an AI-CAD program and radiologists in assessing mammographic density and breast cancer abnormality scores. The first study compared assessments from the AI-CAD program with those from radiologists and an automated assessment program using data from 488 patients at Yongin Severance Hospital, while the second study retrospectively evaluated breast cancer abnormality scores and AI-CAD false-negative cases in 896 patients with 930 breast cancers.
Key findings from the first study include:
● Fair agreement between AI-CAD and radiologists in assessing mammographic density.
● Similar agreement between radiologists and a commercial automated density program.
Implications for AI-CAD application to digital mammograms include:
● Improved Screening Outcomes: AI-CAD enhances radiologists' specificity without compromising sensitivity, particularly beneficial for women with dense breasts or undergoing prevalent screening.
● Agreement with Radiologists: AI-CAD shows fair agreement with radiologists in assessing mammographic density, aiding in breast cancer risk assessment.
● Augmented Categorization and Cancer Detection: AI-CAD correlates with elevated abnormality scores, aiding in BI-RADS categorization, identifying additional cancers missed by radiologists.
● Need for Tailored Algorithms: AI-CAD systems should be designed and trained with population-specific algorithms.
● Limited Occurrence of Cancers: Further studies are needed to confirm AI-CAD effectiveness due to the limited occurrence of cancers in actual screening data.
In summary, AI-CAD has the potential to enhance digital mammography screening accuracy and efficacy in conjunction with radiologists, but further research is needed to validate its effectiveness in different populations.
3.6 AI in breast cancer screening reviews
The comparison of CAD use in screening mammography for early breast cancer detection shows varying effects on diagnostic accuracy and recall rates. Studies comparing single reading (read by one radiologist) (SR) with SR plus CAD showed higher sensitivity and/or cancer detection rates with CAD. However, the addition of CAD to SR generally increased the relative risk (RR) and reduced specificity, except in one study. When comparing double reading (read by two radiologists) (DR) with SR plus CAD, there were no significant changes in sensitivity or cancer detection rates. In most cases, adding a CAD system to screening mammography increased the RR, sensitivity and cancer detection rates.
The advantages of temporal analysis in detecting and classifying breast abnormalities using prior mammogram data include providing a clear benefit in identifying breast masses and microcalcifications by leveraging the comparison with prior mammogram data to aid in the detection and classification of abnormalities. However, a limitation arises when a newly developed abnormality lacks sufficient prior mammogram data for comparison, rendering the temporal analysis less effective in such cases. Additionally, while the integration of prior mammogram data holds significant potential for detecting breast abnormalities, the limited scope of large-scale studies restricts the broad clinical applicability of these findings. Therefore, while temporal analysis using prior mammogram data can be beneficial in certain cases, its effectiveness may be constrained by the availability of relevant historical data.
It is important to note that while most studies showed improvements in sensitivity and cancer detection rates with the addition of CAD, there were concerns about reduced specificity and increased RR in some cases. This indicates that while CAD systems may improve the detection of abnormalities, it may also lead to increased false positives.
4. Discussion
CAD systems show a promising future for enhancing mammography interpretation and breast cancer screening. Their integration into radiology practices could significantly improve diagnostic accuracy and recall rates. However, successful implementation requires further research to optimize CAD integration, consider radiologists' perspectives, and ensure acceptance in clinical settings.
Several challenges persist in CAD and breast cancer diagnosis, notably the low contrast between normal and malignant tissues, especially in dense breasts. Despite promising average performance, CAD methods are currently insufficient for standalone clinical use. Significant improvements through advancements in deep learning and computational power are needed to elevate CAD systems to primary diagnostic tools.
The efficacy of breast cancer detection depends on the CAD system's performance, the tested population, and radiologists' expertise. CAD can particularly benefit less experienced radiologists by aiding in the identification of tumors with microcalcifications. Understanding CAD's clinical integration and its impact on healthcare practitioners remains crucial, necessitating further research. Evaluating the costs associated with CAD systems is also imperative for optimizing their healthcare applications. The integration of prior mammogram data in AI tools shows significant potential but is currently limited by the lack of large-scale studies. Initial adoption in clinical settings may serve as a supplementary tool for a second reader. Most studies demonstrate improved recall rates, sensitivity, and cancer detection rates with CAD, indicating its potential for enhanced diagnostic accuracy.
Table 1. Interpretation of BIRADS Categories in Mammographic Imaging Evaluations
5
|
Category
|
Category Clinical interpretation
|
| Category 0 |
Incomplete, further imaging evaluations are required |
| Category 1 |
Negative, no abnormality found |
| Category 2 |
Benign |
| Category 3 |
Probably benign |
| Category 4 |
Suspicious finding |
| Category 5 |
Highly suggestive of malignancy |
| Category 6 |
Known biopsy-proven malignancy |
Table 2. Search queries for each database consulted
|
Database
|
Search string
|
| Database |
Search Query |
| SCOPUS |
CAD AND Mammography |
| EBSCO |
CAD AND Mammographies AND BI RADS |
| PubMed |
CAD AND Mammographies |
Table 3. CAD performance in analyzing mammograms
| Study |
Task |
Method |
Dataset |
Comparison |
Results |
| Al-masni, M., et al. in 2018 |
Segmentation and classification of benign/malignant lesions. |
CNN, YOLO-based CAD system. |
DDSM (18) |
Support Vector Machine, Probabilistic Neural Network, CNN. |
-Accuracy: 99.7% for mass detection and 97% distinguishing between benign and malignant lesions.
-Sensitivity: 100% for benign cases.
-Specificity: 94% for malignant cases
-F1 score: Not reported.
-MCC: Not reported
|
| Al-antari, M., et al. in 2018 |
Segmentation and classification of benign/malignant lesions. |
Deep learning model based on a FrCN and CNN both with YOLO approach and to evaluate the INbreast database is used. |
INbreast dataset (7) |
YOLO, FrCN, CNN. |
No segmented mass.
INbreast with mass segmentation via four-fold cross-validation
-Accuracy: 95.64%
-Sensitivity: 97.14%
-Specificity: 92.41%
-F1 score: 96.84
-AUC: 94.78%
-MCC: 89.91%
|
| Assari, Z., et al. in 2022 |
Solid breast mass classification |
Training separate models for each imaging modality, then integrating them into a single BCNN |
DDSM, BUSI, Collected Dataset (9) |
Sonographic Monomodal Model (SMCNN) and Mammographic Monomodal Model (MMCNN) |
-Accuracy: 90.38%
-Sensitivity: 90.91%
-Specificity: 89.87%
-F1 score: 90.32
-MCC: 80.78%
-SE: 90.91%
-SP: 89.8
-AUC: 95.82%
|
| Boumaraf, S., et al. in 2020 |
Classify mammographic masses according to BI-RADS. |
A modified Genetic Algorithm (GA) is used for feature selection in classifying breast masses in mammograms, with a back-propagation neural network (BPN) for classification. |
DDSM (5) |
Traditional CAD system, BI-RADS classification |
-Accuracy: 84.5%
-Sensitivity: 84.5%
-Specificity: 94.25
-F1 score: Not reported
-MCC: 79.3%
-PPV: 84.4%
-NPV: 94.8%
|
| Chougrad, H., et al. in 2018 |
Segmentation and classification of benign/malignant lesions. |
Transfer learning with pre-trained models to extract features and fine-tune them to distinguish between malignant and benign lesions. |
DDSM, INbreast, BCDR and The Merged Dataset, independent database MIAS (6) |
Human performance, radiologists. |
-Accuracy
-DDSM: 97.35%
-INbreast: 95.50%
-BCDR: 96.67%
-Breast Cancer Screening Framework: 98.94%
-MIAS: 98.23%
-AUC
-DDSM: 0.98
-INbreast: 0.97
-BCDR: 0.96
-Breast Cancer Screening Framework: 0.99
-MIAS: 0.99
-Sensitivity: Not reported
-Specificity: Not reported
-F1 score: Not reported
-MCC: Not reported
|
| Baccouche, A., et al in 2022 |
Segmentation and classification of benign/malignant lesions. |
Comparative experiments were performed on both individual models and an average ensemble of models using an XGBoost classifier. |
CBIS-DDSM, INbreast and a private dataset (19) |
Mammographies datasets CBIS-DDSM, INbreast and private dataset. |
-Accuracy by:
-Pathology classification
-CBIS-DDSM: 95.13%
-INbreast: 99.20%
-Private dataset: 95.88%
-BIRADS category
-CBIS-DDSM: 85.38%
-INbreast: 99%
-Private dataset: 96.08%
-Shape classification
-CBIS-DDSM: 90.02%
-AUC by:
-Pathology classification
-CBIS-DDSM: 0.95
-INbreast: 0.99
-BIRADS category
-CBIS-DDSM: 0.94
-INbreast: 1.0
-Sensitivity by:
-CBIS-DDSM: 0.85
-INbreast: 1.0
-Specificity by:
-CBIS-DDSM: 0.98
-INbreast: 0.96
-F1 score:
-CBIS-DDSM: 0.91
-INbreast: 0.99
|
| James, J., et al. in 2018 |
Evaluate CAD-enhanced 2D synthetic mammograms vs standard synthetic mammograms and full-field digital mammography (FFDM) for diagnostic performance. |
Two radiologists, blinded to image type and final assessment, retrospectively reviewed oblique and craniocaudal mammogram projections. |
FFDM, Standard synthetic mammograms generated from digital breast tomosynthesis (DBT) data, and CAD-enhanced synthetic mammograms (20) |
Standard synthetic 2D mammograms and conventional 2D FFDM. |
-CAD-enhanced synthetic mammogram
-AUC: 0.846
-Sensitivity: 94.11
-Specificity: 57.35
-Standard synthetic mammogram
-AUC: 0.683
-Sensitivity: 52.94
-Specificity: 86.76
-Conventional 2D FFDM
-AUC: 0.724
-Sensitivity: 97.06
-Specificity: 17.64
|
Table 4. Analysis of current advancements and challenges faced in CAD
| Author |
Objective |
Reported results |
Conclusions |
Observations |
| Ramadán S in 2020 |
The paper reviews recent advancements in CAD systems for breast cancer detection and diagnosis via mammograms, outlining methods utilized in CAD systems and addressing the demand for breast imaging specialists. It emphasizes the importance of screening mammography for early detection of breast cancer and highlights associated challenges (10). |
Reported advancements and current state of CAD for breast cancer detection show that, despite promising performance levels, CAD systems are not yet reliable enough to be used independently for detecting and diagnosing breast cancer with mammograms.
The average performance of various CAD methods, measured by the area under the ROC curve, is around 0.86. However, the excellent performance results in literature cannot be generalized, as they are based on specific datasets. |
Computer-aided detection and diagnosis of breast cancer from mammograms face challenges due to low contrast between normal and malignant tissues, especially in dense breast tissue.
The average performance of CAD methods is encouraging but falls short of reliability for standalone clinical use.
Without significant improvement, particularly through leveraging deep learning and enhanced computational power, CAD systems are deemed suitable only as secondary opinion tools in clinical practice. |
No PRISMA methodology was presented. Article is more centered about the technological state of CAD than the clinical application. |
| Guo Z et al. in 2022 |
This paper explores the application of CAD systems for breast cancer detection, focusing on their role in breast imaging and the integration of AI in radiology. It assesses the effectiveness of CAD in screening mammography and delves into the potential of CAD using image mining techniques for breast cancer screening. Additionally, it reviews methodologies in CAD systems for breast cancer detection, aiming to offer a comprehensive understanding of CADs utilization in breast cancer diagnosis and screening (21). |
The study found that MRI had the highest sensitivity, while MM had the lowest, regardless of breast type, density, or history. Sensitivity increased with combined modalities such as US + MRI or MM + MRI or MRI + MM + US. Additionally, conventional CAD systems accuracy has been enhanced with the introduction of AI and AI-based algorithms. |
Breast cancer detection efficacy relies on the CAD system, the targeted population, and radiologists expertise.
CAD aids inexperienced radiologists, particularly in identifying microcalcification presenting carcinomas. As AI progresses, understanding CADs clinical integration and its effects on practitioners becomes vital, warranting further research.
Additionally, evaluating CAD system costs for breast carcinoma screening is essential for streamlining their healthcare applications. |
Study directly compared the application of CAD with different imaging modalities with mammograms. |
Table 5. CAD models performance in varying imaging modalities
| Study |
Task |
Method |
Dataset |
Comparison |
Results |
| Patel B et al. in 2018 |
Development and evaluation of a prototype CAD tool for contrast-enhanced spectral mammography (CESM). |
Developed a prototype CAD-CESM tool using texture and morphologic analysis to differentiate benign from malignant breast lesions. |
CESM (12) |
CAD with 2 experienced radiologists |
CAD CESM:
- Accuracy: 90%
- Detection rate for the malignant group: 88%
- Detection rate for the benign group: 92%
RADIOLOGIST 1:
- Accuracy: 78%
- Detection rate for the malignant group: 92%
- Detection rate for the benign group: 62%
RADIOLOGIST 2:
- Accuracy: 86%
- Detection rate for the malignant group: 100%
- Detection rate for the benign group: 71%
F1 Score: Not reported
MCC: Not reported
|
| He Z et al. in 2021 |
Evaluate the diagnostic performance of a CAD model for breast masses. |
In the MRMC study, we selected six specialists of radiology into three groups, the radiologists diagnosed 51 patients with and without the CAD model. |
FFDM images (11) |
Six radiologists with CAD and without CAD |
UNAIDED
SENSITIVITY
Reader 1: 0.545
Reader 5: 0.819
SPECIFICITY
Reader 2: 0.621
Reader 6: 0.863
PPV
Reader 1: 0.857
Reader 2: 0.577
NPV
Reader 2: 0.720
Reader 5: 0.851
AUC
Reader 2: 0.783
Reader 3: 0.889
AIDED
SENSITIVITY
Reader 1: 0.682
Reader 5: 0.901
SPECIFICITY
Reader 1: 0.931
Reader 2: 0.621
PPV
Reader 1: 0.882
Reader 2: 0.633
NPV
Reader 1: 0.794
Reader 5: 0.909
AUC
Reader 3: 0.922
Reader 5: 0.847
|
| Yoon J et al. in 2022 |
Evaluate an AI-CAD system's performance in assessing the malignancy risk of calcifications detected on screening mammography. |
Retrospective diagnostic or prognostic study performed at one institution. |
AI-CAD system (22) |
AI-CAD system and an experienced breast radiologist. |
Accuracy:
AI CAD: 80%
Radiologist: 81.7%
Sensitivity
AI CAD: 92.1%
Radiologist: 95.4%
Specificity
AI CAD: 90.3%
Radiologist: 96.9%
|
| Dominkovic M et al. in 2020 |
Compare CAD analysis with two radiologists' independent assessments for screening mammography. |
Radiologists reviewed the images and classified the findings according to the Breast Imaging Reporting and Data System category. |
CAD system (23) |
CAD in screening mammography with two independent radiologists with the same sample. |
Accuracy: Not reported
ALL LESIONS:
- Radiologists: 89 (100%)
- CAD: 49 (54%)
- Sensitivity: 54%
- Specificity: 16%
- PPV: 23%
- NPV: 37%
LESIONS WITHOUT ALN:
- Radiologists: 69 (100%)
- CAD: 29 (42%)
- Sensitivity: 47%
- Specificity: 16%
- PPV: 19%
- NPV: 43%
LESIONS WITHOUT BENIGN MASSES AND ALN:
- Radiologists: 18 (100%)
- CAD: 8 (44%)
- Sensitivity: 44%
- Specificity: 16%
- PPV: 6%
- NPV: 71%
SUSPICIOUS LESIONS:
- Radiologists: 7 (100%)
- CAD: 6 (86%)
- Sensitivity: 85%
- Specificity: 16%
- PPV: 5%
- NPV: 96%
F1 SCORE: Not reported
|
| Watanabe A et al. in 2019 |
Assistive detection of breast cancer in 2D FFDM |
cmAssist™, an AI-CAD |
Health Insurance Portability and Accountability Act (HIPAA)-compliant protocol (2) |
Radiologists using the R2 ImageChecker CAD, version 10.0. In the reader study, 7 radiologists. |
Accuracy: Not reported
Sensitivity: Not reported
Specificity: Not reported
F1 SCORE: Not reported
AUC: 7.2%
CDR with the use of cmAssist: 62%
CDR without cmAssist: 51%
CI: 1.14% A 15%
|
Table 6. Current performance results of AI in evaluation of lesions
|
Author
|
Objective
|
Reported results
|
Conclusions
|
Observations
|
| Jairam M et al. en 2021 |
Examine the part of AI instruments in both advanced mammography and DBT (3). |
AI-supported disease diagnosis enhances diagnostic accuracy compared to traditional CAD systems. It presents a promising standalone diagnostic method, potentially substituting the need for a second reader. Through the identification and deprioritization of negative mammograms, deep learning tools streamline radiologists workload. |
AI is poised to advance as a supportive instrument in breast imaging, offering significant potential for enhancing breast cancer detection and diagnosis. |
The article delineates various studies and elucidates their distinct findings. It does not provide specificity or sensitivity data. |
| Masud R et al. 2019 |
Provide an overview of current research regarding the integration and application of CAD in breast cancer screening by radiologists, highlighting obstacles and aids in CAD adoption (24). |
Factors promoting CAD utilization included enhanced breast cancer detection rates, heightened financial viability in breast imaging, and time efficiency gained through replacing dual interpretations. |
Additional investigation is warranted to identify optimal strategies for integrating CAD into radiology workflows to enhance patient results, emphasizing the importance of incorporating radiologists' perspectives in advancing CAD utilization. |
The article employs retrospective analysis to examine and ascertain the extent of implementation and associated expenses. |
| Retson T et al. en 2023 |
Delve further into the various ways AI is applied in breast imaging, exploring its wide range of applications (14). |
Future efforts will focus on developing techniques for radiologists to interpret AI-generated decisions, aiming to enhance radiology practices' efficiency and improve patient care. |
It is essential to address the ongoing updates and maintenance of AI algorithms to maintain peak performance, considering potential alterations stemming from software updates or demographic shifts. |
Article includes no sensitivity or specificity. |
| Hamidinekoo A et al. in 2018 |
It delineates the correlation between mammography findings and histopathological phenotypes, considering biological factors (15). |
Mammographic abnormalities characteristics can be indicative of specific histological details, offering insights into how microscopic changes manifest in macroscopic images. |
Various imaging modalities provide diverse information across different scales, aiding in estimating breast cancer stage and risk. Clinicians integrate these varied data sources to enhance disease diagnosis and treatment planning. |
The proposed Mammography-Histology-Phenotype-Linking-Model aims to establish a connection between features/phenotypes observed in mammographic abnormalities and their corresponding histopathological representations. |
| Jones M et al. in 2022 |
Observe recent advances in understanding the relationship between radiomic features and the tumor microenvironment, as well as the progress in developing new AI-based quantitative image analysis models in three key areas of breast cancer diagnosis (4). |
Radiomics and deep transfer learning methods show promise in extracting clinically relevant image features to create quantitative markers and prediction models for breast cancer research. This AI-driven approach is advancing personalized medicine by using patient-specific data for cancer detection and diagnosis. |
Advances in high-throughput radiomics and AI-based deep transfer learning have led to numerous new CAD schemes and prediction models for breast cancer, including cancer risk prediction, tumor malignancy likelihood, tumor subtypes or staging, treatment response, and patient survival outcomes. However, these new AI-based CAD schemes require further validation with large, diverse image databases from multiple clinical sites before clinical adoption. |
More research is needed for a conclusive answer. |
| Arce S et al. in 2023 |
Emphasize recent advancements in understanding the relationship between the radiomics features and the tumor microenvironment, as well as the development of novel AI-driven quantitative models for analyzing image features in three domains of breast cancer (16). |
Investigated terms encompassing breast cancer risk, diagnosis/classification, CAD, and treatment response/prognosis prediction. |
Beyond commercially available CAD schemes, progress in technologies such as high-throughput radiomics feature analysis and AI-driven deep transfer learning has spurred the creation of numerous new CAD schemes. |
All patients in this dataset had cancer, the specificity of the AI CAD could not be calculated, and a receiver operating characteristic analysis could not be performed. |
| Bahl M in 2018 |
Evaluate the possible enhancement in performance measures like sensitivity, specificity and AUC when radiologists receive assistance from an AI system in interpreting mammograms (13). |
Their mean AUC increased from 0.87 to 0.89 with AI assistance. Sensitivity showed improvement with AI aid (86% vs. 83%), and specificity demonstrated a favorable trend (79% vs. 77%). |
Incorporation of AI systems into standard clinical practice could potentially assist radiologists of varying expertise levels in achieving performance standards. |
While AI systems based on deep learning, such as Transpara, hold promise in aiding radiologists in mammography-based breast cancer detection, the enhancements in performance with AI assistance remain marginal. |
| Kohli A et al in 2017 |
Examine the evolution of CAD in mammography, assess the reasons behind CADs shortcomings, and speculate on potential strategies for future CAD systems to achieve success (25). |
CAD showed limited efficacy in enhancing cancer detection accuracy. Despite a marginal increase in cancer detection rates (ranging from 2% to 10%) and a slight acceleration in cancer detection by 2 months, it did not result in improved outcomes. |
The endeavor to detect breast cancer through CAD has encountered obstacles and constraints. |
No PRISMA methodology was presented. |
Table 7. AI-CAD evaluation for breast cancer screening
|
Study
|
Goal
|
Method
|
Dataset
|
Comparison
|
Reported results
|
| Lee S et al. in 2022 |
Evaluate agreement between an AI-CAD program and radiologists in assessing mammographic density, important for breast cancer risk assessment (26). |
The study compared breast density assessments from a commercial AI-CAD program with those from radiologists and an automated assessment program, using data from 488 patients at Yongin Severance Hospital. |
At Yongin Severance Hospital, a study compared mammogram assessments from an automated density program and an AI-CAD program for 488 patients. This dataset is under license and is not publicly available. |
An automated density assessment program AI-CAD with the assessments made by radiologists. |
Density assessment results underscore the need for population-specific algorithms. AI-CAD density assessments showed fair agreement with radiologists, comparable to the commercial programs. |
| Lee S et al. in 2022-1 |
Evaluate breast cancer abnormality scores based on clinical, radiological, and pathological characteristics (17). |
Study retrospectively evaluated breast cancer abnormality scores and AI-CAD false-negative cases. Included 896 patients with 930 breast cancers. Commercial AI-CAD applied to digital mammograms. |
Mammograms of 896 patients with breast cancer. |
Abnormality score of the AI-CAD algorithm and the clinical, radiological, and pathological characteristics of breast cancer. |
BI-RADS 0 and BI-RADS 3 were not reported, due to the study focusing only on pre-reviewed mammograms with cancer. Analysis revealed a correlation between breast cancers characterized by elevated abnormality scores on AI-CAD and augmented BIRADS categorization, invasive pathological features, and advanced cancer staging. |
| Kim H et al. in 2023 |
Evaluate AI-CADs impact on breast cancer screening outcomes with digital mammography (27). |
A retrospective analysis of data from asymptomatic Korean women who underwent screening DM with and without AI-CAD support. |
Histopathologic results from surgery and imaging-guided biopsy, as well as stability at follow-up imaging. This dataset is under license and is not publicly available. |
Performance of radiologists with and without the support of AI-CAD in digital mammography screening. |
AI-CAD enhances radiologists' specificity without sacrificing sensitivity, working as a supportive tool in single reading DM for breast cancer screening, especially useful in interpreting DM for women with dense breasts or those undergoing prevalent screening. Further studies with a larger number of cases are necessary to confirm AI-CAD's true effectiveness on CDR and sensitivity due to the limited occurrence of cancers in actual screening data. |
| Yoon et al. in 2023 |
Evaluate outcomes of abnormalities detected by AI-CAD and its stand-alone diagnostic performance in a screening population (28). |
A retrospective, cross-sectional design. The study included 6499 four-view, full-field digital mammograms of 5,228 women who had undergone biopsy for pathological diagnosis, or a round of screening. |
6499 four-view, full-field digital mammograms from 5228 women who had undergone biopsy or additional screening at the facility. |
Performance of radiologists and an AI-based diagnostic support software in the interpretation of mammography images for breast cancer detection. |
AI-CAD identified additional cancers initially missed by radiologists in a consecutive screening population. Despite this, stand-alone AI-CAD resulted in notably higher recall rates compared to radiologists' interpretations, with 89% of AI-CAD marks being confirmed as negative. |
Table 8. Advances in automated detection for breast abnormalities
|
Author
|
Objective
|
Reported results
|
Conclusions
|
Observations
|
| Loizidou K et al. in 2022 |
Review recent advances in automated detection and classification of breast abnormalities using sequential mammograms, focusing on prior information use and guiding future applications. (29) |
Temporal analysis provides a clear advantage in detecting and classifying breast masses and microcalcifications (MCs). However, this technique does not offer benefits when a newly developed abnormality lacks sufficient prior mammogram data for comparison. |
The integration of prior mammogram data holds significant potential for detecting breast abnormalities, nonetheless, the limited scope of large-scale studies restricts the broad clinical applicability of these findings. |
Slow adoption in clinical settings may initially confine temporal subtraction to a supplementary role for a second reader. Authors focused more on the technological aspects of the tools and little information was found regarding clinical applications. |
| Henriksen E et al. in 2019 |
Compare CAD use in screening mammography for early breast cancer detection, focusing on diagnostic accuracy and recall rates. (1) |
Most studies comparing SR to SR and CAD showed higher sensitivity and/or cancer detection rates when in use with CAD. Comparing DR to SR with CAD revealed no significant changes in sensitivity or detection rates. Adding CAD to SR increased RR and reduced specificity, except in one study. |
All but two studies showed that adding CAD to SR increased RR, sensitivity, and cancer detection rate (CDR). Compared to DR, no statistically significant differences in sensitivity or CDR were observed. |
Review followed and provided respective PRISMA guidelines, and analyzed and compared 13 different studies. Further studies are needed to correctly evaluate CAD efficacy in organized population-based screening programs, with longer follow-up, high-volume readers, and digital mammography. |
| Loizidou K et al. in 2023 |
Detect and classify breast abnormalities, including masses and microcalcifications. (30) |
The authors do not provide a global evaluation of what the results reported by each paper could mean. |
This review covers mammogram role in detecting breast masses and MCs but acknowledges the need for other imaging modalities. More research is needed to validate CAD algorithms with ample clinical data. Despite limitations, this review offers valuable insights for future studies. |
Article presents a detailed analysis of the current state of CAD in mammography and its potential clinical application. Authors focused on the technical aspect. |
Figure 1
PRISMA diagram of the literature review stage of the project
Conclusión
Further research, especially in organized population-based screening programs with longer follow-up times and digital mammography, is necessary to fully assess CAD efficacy. Validation of CAD algorithms with ample clinical data remains a critical area for future research and improvement.