INTRODUCTION
Hypercalcemia, defined as a serum calcium level above 10.5 mg/dL or ionic calcium above 5.6 mg/dL, is a rare and potentially serious metabolic disorder. In most cases, it is caused by parathyroid disorders and neoplasms
1
. The clinical presentation is closely related to blood calcium levels and can range from asymptomatic to severe neurological, muscular, gastrointestinal, renal, and cardiovascular manifestations
2
.
In calcium homeostasis, vitamin D plays a crucial role
3
. However, its excessive and unsupervised administration can lead to complications such as hypercalcemia, hypercalciuria, hyperphosphatemia, among others
4
. Although hypercalcemia secondary to vitamin D is rare, there has been a reported increase in its prevalence, likely due to the rise in the use of supplements following the awareness of various chronic diseases associated with vitamin D deficiency
5, 6
.
This case describes an elderly woman presenting with hypercalcemia and initially nonspecific clinical symptoms. After ruling out the most common organic causes, vitamin D intoxication was determined as the etiology, a rarely seen condition in our environment. This report highlights the importance of responsible vitamin D supplementation in everyday medical practice and the need for appropriate clinical monitoring.
Caso Clínico
An 86-year-old woman, born in Lima, experienced progressive hyporexia one month before her emergency admission. Two days before, she presented with muscle weakness, nausea, vomiting, hypersalivation, and polyuria, and on the day before admission, confusion and incoherent speech were added.
The patient had no known drug allergies and a medical history of type 2 diabetes mellitus, systemic arterial hypertension, and osteoarthritis. Her regular medications included metformin, enalapril, and diltiazem. Three months before admission, she underwent hip arthroplasty, and from that time, vitamin D (cholecalciferol) supplements at a dose of 5000 IU every eight hours were added to her medication regimen.
Upon arrival in the emergency department, the patient appeared pale and thin. Her temperature was recorded at 37.2 °C, blood pressure at 170/110 mmHg, heart rate at 90 beats/min, respiratory rate at 25 breaths/min, and oxygen saturation at 93% on room air. Respiratory evaluation revealed basal crackles in the right hemithorax, while neurological examination showed somnolence, confused speech, and disorientation in time and space. The rest of the physical examination was normal.
Blood tests showed hemoglobin at 10.5 g/dL, white blood cells at 12,420/mm3 with 86.2% (10,950) neutrophils and 6.1% (760) lymphocytes, along with a C-reactive protein of 7.03. Arterial blood gas analysis revealed a pH of 7.45, pCO2 of 26.3 mmHg, and HCO3 of 18.1 mEq/L (compensated metabolic alkalosis). Electrolytes showed potassium at 3.38 mEq/L, sodium at 143 mEq/L, ionic calcium at 7.27 mg/dL, and chloride at 115.7 mEq/L. Blood glucose was 247.3 mg/dL, creatinine was 0.83 mg/dL, and lactate was 1.33 mmol/L. The chest X-ray showed no abnormalities. Therefore, the case was approached as a patient with pneumonia with probable respiratory failure and metabolic origin sensorium disorder. Treatment was initiated with ceftriaxone at a dose of 2 g every 24 hours, intravenous hydration with isotonic saline (80 mL/h), furosemide 20 mg every eight hours, nifedipine 30 mg every 24 hours, and regular insulin on a sliding scale.
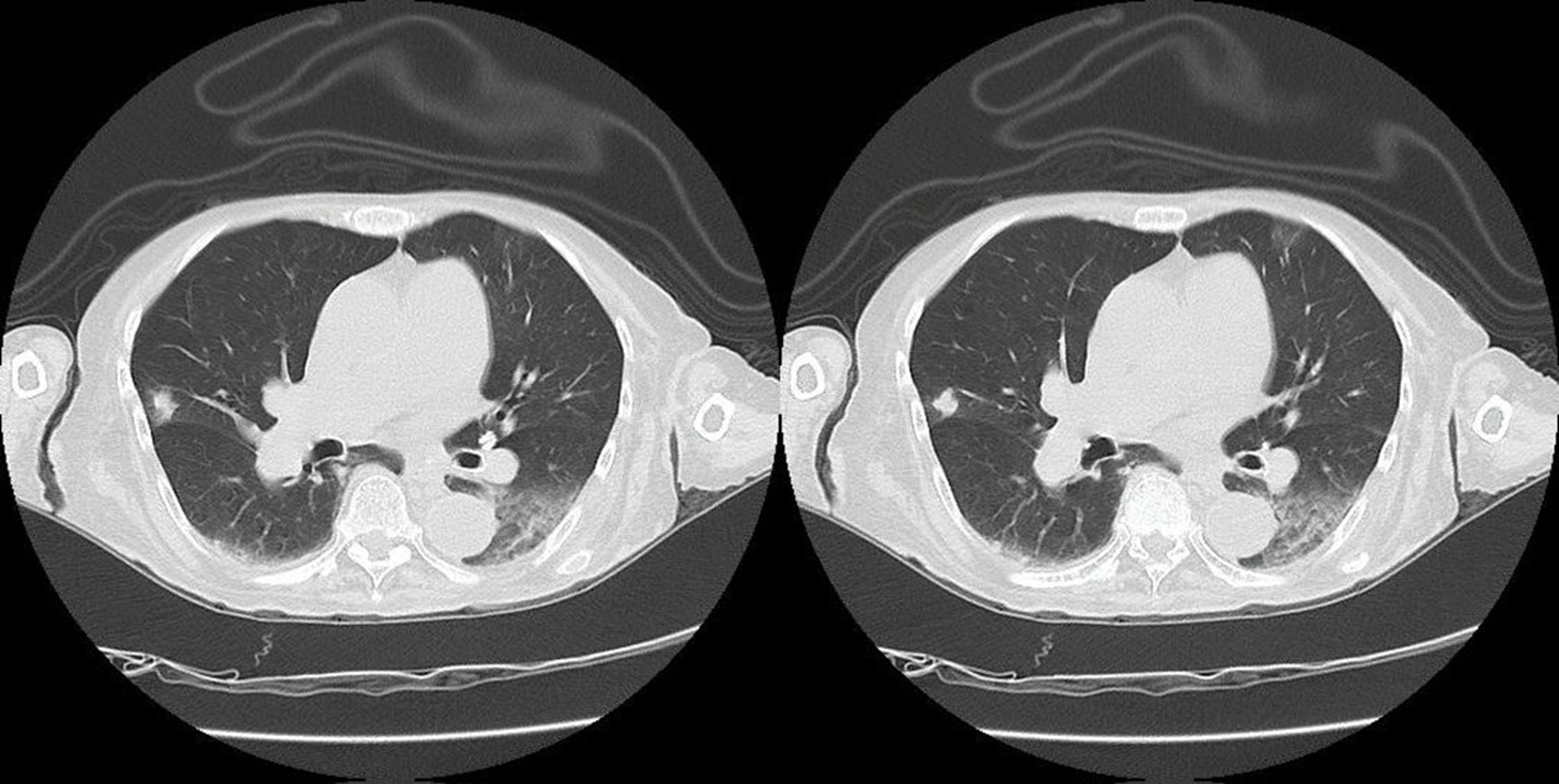
The patient was hospitalized for further investigation of hypercalcemia. Initially, parathyroid pathology was suspected, and a soft tissue cervical ultrasound was performed, revealing a 4 mm spongiform cyst in the lower thyroid lobe. Additionally, serum parathyroid hormone and calcitonin levels were measured, both within normal ranges, thus ruling out this cause. Subsequently, neoplastic etiology was suspected, so oncological markers were requested, yielding normal values, and a CT scan of the head, chest, abdomen, and pelvis was ordered, which revealed an increase in the interstitium in both lower lobes, an 8 mm subcisural nodular lesion in the middle lobe, and a left laminar pleural effusion
(figura 1).
Figure 1
Axial slices of thoracic CT in pulmonary window showing interstitial involvement in both lower lobes, a nodular lesion in the middle lobe, and a left laminar pleural effusion.
Source: Own elaboration.
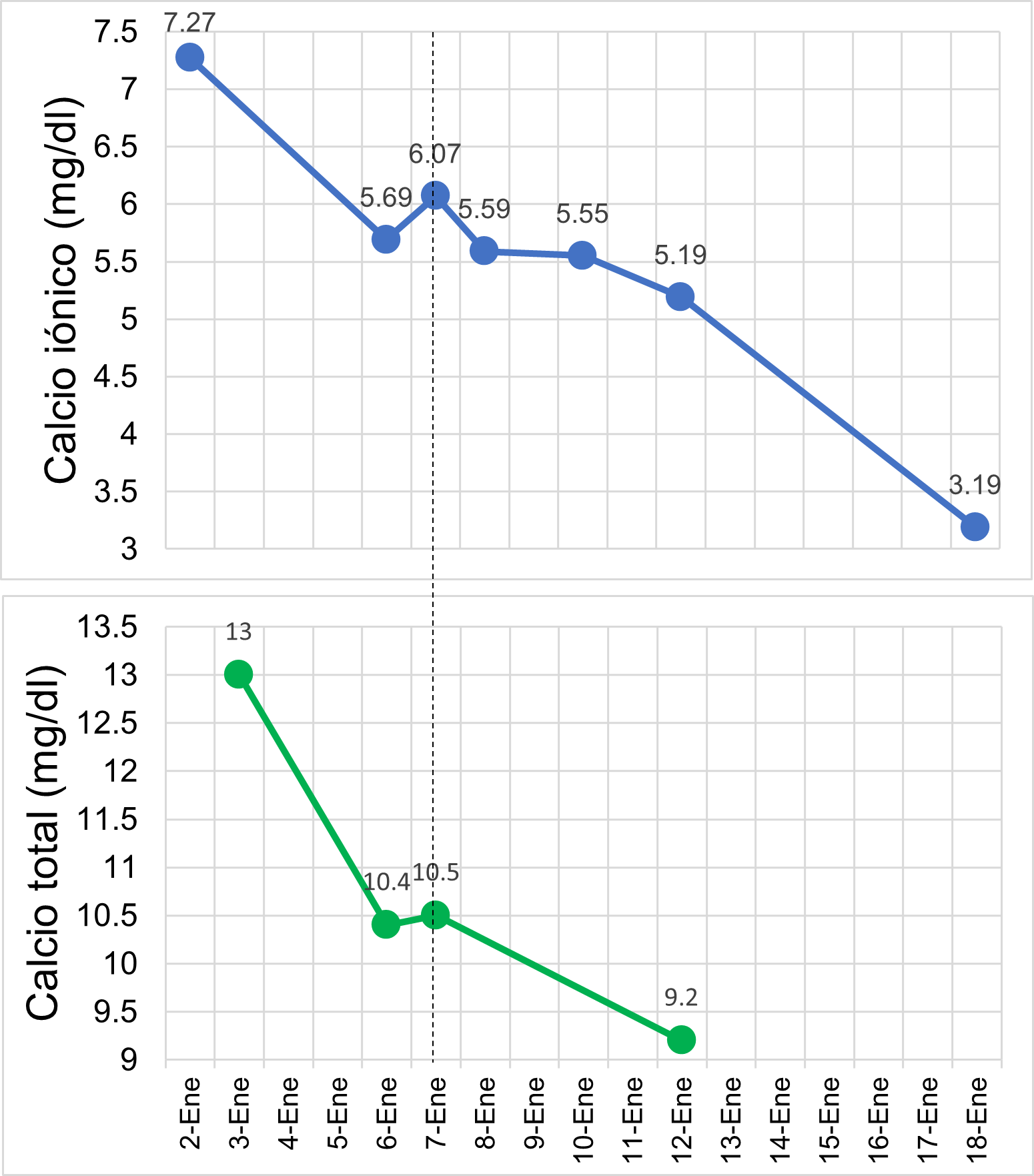
On the second day, ionic calcium was found to be 5.69 mg/dL, and potassium was 1.73 mEq/L, leading to the suspension of furosemide and the initiation of treatment with hydrocortisone at 100 mg every 12 hours, along with intravenous potassium correction. On the third day, ionic calcium levels increased to 6.07 mg/dL. In parallel, the patient experienced further cognitive decline, temporal disorientation, and displayed aggressive behavior. In response, pamidronate was administered in a single dose of 90 mg intravenously.
Since auxiliary exams ruled out organic causes of hypercalcemia, it was suspected that the patient developed vitamin D intoxication. Therefore, on the fifth day of hospitalization, a 25-hydroxyvitamin D assay was requested, which revealed a value greater than 150 ng/mL, confirming vitamin D intoxication as the underlying cause. One week later, normalization of calcium levels was observed (figura 2), and the patient showed favorable clinical progress, with a gradual recovery of consciousness. Consequently, the patient was discharged on the 18th day of hospitalization.
Figure 2
Evolution of ionized and total calcium levels.
Fuente: Own elaboration.
DISCUSSION
This case involves an elderly patient diagnosed with hypercalcemia secondary to vitamin D intoxication. Worldwide, the prevalence of hypercalcemia ranges from 0.2% to 4% Se presenta el caso de una paciente adulta mayor con diagnóstico de hipercalcemia secundaria a una intoxicación por vitamina D. A nivel mundial, la prevalencia de la hipercalcemia oscila de 0.2 % a 4 %This case involves an elderly patient diagnosed with hypercalcemia secondary to vitamin D intoxication. Worldwide, the prevalence of hypercalcemia ranges from 0.2% to 4%
3
. Hypercalcemia associated with cancer and primary hyperparathyroidism are the most frequent causes
1
,
3
. However, there are other rare causes, such as vitamin D intoxication, for which a probable increase in prevalence has been reported following higher intake and prescription of vitamin D supplements
6
.
Protocols regarding the administration of vitamin D are heterogeneous, with doses ranging from 800 to 2000 IU per day
7
. Furthermore, the maximum safe dose is unknown; some studies describe values between 4000 and 10,000 IU/day as the maximum safe dose
1
,
7
. However, recent studies have shown that the consumption of 2345 IU/day was associated with a 50% increase in hypercalcemia, approximately
1
. Cases of intoxication have been reported with doses ranging from 15,000 to 100,000 IU/day
8
,
9
. Our patient received a dose of 15,000 IU/day for 3 months, resulting in a cumulative dose of 1,350,000 IU.
Vitamin D intoxication typically occurs in the elderly population, as most protocols suggest supplementation in this group
10
,
11
. Our patient, an elderly woman, consumed vitamin D according to medical advice after undergoing hip surgery.
Regarding the clinical presentation and findings, they are closely related to the serum concentration and duration of hypercalcemia
3
,
4
. Clinical manifestations include neuropsychiatric symptoms such as confusion, stupor, or coma; gastrointestinal symptoms like abdominal pain, vomiting, anorexia, constipation; cardiovascular symptoms such as hypotension, bradyarrhythmias, short QT interval, among others; and renal symptoms like hypercalciuria, acute kidney injury, dehydration, and nephrocalcinosis
1
. In this case, the patient initially presented with hyporexia, which progressed to anorexia, muscle weakness, polyuria, vomiting, and confusion. There was no progression to acute kidney injury, and creatinine levels remained within normal ranges. No other severe manifestations occurred.
After ruling out endocrine and neoplastic causes, the diagnosis of hypercalcemia due to vitamin D intoxication is determined by serum 25-hydroxyvitamin D levels above 100-150 ng/mL
1
,
12
,
13
. In our patient, the level was reported as greater than 150 ng/mL, confirming vitamin D intoxication as the underlying cause of hypercalcemia.
The treatment of hypercalcemia depends on its severity, duration, and symptoms. Patients with asymptomatic, mild hypercalcemia (total calcium <12 mg/dL or ionic <8 mg/dL), moderate hypercalcemia (total calcium <14 mg/dL or ionic ≤10 mg/dL), and chronic hypercalcemia typically do not require immediate treatment
1
. Severe hypercalcemia (total calcium ≥14 mg/dL or ionic >10 mg/dL) affects cardiac, central nervous system, renal, and gastrointestinal functions, requiring immediate management (1, 14).
1
,
14
. Management strategies include hydration with isotonic solution (200-300 mL/h) and the use of drugs such as loop diuretics, bisphosphonates, glucocorticoids, and calcitonin; in extreme cases, dialysis is considered
1
,
15
. Additionally, the main goal in treating vitamin D-dependent hypercalcemia is to discontinue its intake.
The patient’s total and ionic calcium levels upon admission were 13.0 mg/dL and 7.27 mg/dL, respectively. Thus, it was classified as mild symptomatic acute hypercalcemia. Vitamin D administration was discontinued upon admission, and hypercalcemia correction was initiated with saline infusion along with a loop diuretic. Despite this, calcium levels remained elevated, so intravenous hydrocortisone at 100 mg every 12 hours was administered. Given the worsening neurological decline, a single dose of 90 mg pamidronate was administered. Although studies have demonstrated the superiority of zoledronic acid over pamidronate, the latter is preferred in cases of hypercalcemia caused by vitamin D intoxication
1
. The onset time of both medications is estimated between 24-48 hours. In our patient, serum and ionic calcium levels began to decrease 24 hours after administration.
The patient described in this case presented with metabolic alkalosis, hypernatremia, hypokalemia, and hypercalcemia. This combination could be attributed to a complex interaction between the direct effects of hypercalcemia, which include increased diuresis, consequent dehydration, and activation of the renin-angiotensin-aldosterone system (RAAS). Hypercalcemia induces polyuria by interfering with the renal tubules' ability to concentrate urine, leading to excessive loss of water and electrolytes, resulting in dehydration and hypernatremia. Dehydration and secondary hypovolemia activate the RAAS, increasing aldosterone secretion, which promotes sodium reabsorption in the renal tubules and the excretion of potassium and protons; this contributes to hypokalemia and metabolic alkalosis. Additionally, some studies have shown that hypercalcemia can directly stimulate aldosterone release, further exacerbating the mentioned imbalances
16
.
This case presents a rare etiology of hypercalcemia and emphasizes the importance of considering vitamin D intoxication in the differential diagnosis of hypercalcemia, as well as the need to monitor vitamin D levels in patients undergoing supplementation, particularly in the geriatric population. Clear communication between the physician and the patient regarding the safety profile and the risks associated with excessive administration is crucial to avoid severe complications.
CONCLUSION
It is important to consider vitamin D intoxication as a differential diagnosis of hypercalcemia to achieve timely management and prevent potentially serious complications. Monitoring strategies for vitamin D levels in geriatric patients receiving vitamin supplements are recommended to ensure an appropriate dosage and prevent toxicity.