1. INTRODUCTION
Radiotherapy is a therapeutic modality aimed at controlling or eradicating the volume of malignant tumors while preserving as much as possible the healthy cells adjacent to the treated area
1
,
2
. This therapy can be performed using two distinct techniques depending on the position of the radiation source: if the equipment emitting radiation is distant from the patient, it is called teletherapy. However, if the therapy is conducted using radionuclides, which are placed near or in contact with the tumor region, it is termed brachytherapy
3
,
4
. In both treatment modalities, it is important to ensure the equality between the prescribed and delivered dose to the patient, highlighting the importance of dosimetry
5
,
6
,
7
,
8
.
Dosimetry in teletherapy is guided by TRS-398
9
, developed by the International Atomic Energy Agency (IAEA). In contrast, dosimetry in brachytherapy is governed by the TG-43 protocol
10
, later revised as TG-43U1
11
, which is established by the American Association of Physicists in Medicine (AAPM). This formalism recommends the use of Monte Carlo simulation due to the high dose gradient in regions close to the brachytherapy source, making experimental dosimetry highly complex.
Therefore, different Monte Carlo simulation codes can be used to determine the dosimetric parameters responsible for this dosimetry, including: Geant4 Application for Tomographic Emission (GATE)
12
,
13
, Monte Carlo Neutron-Photon (MCNP)
14
,
15
, PENetration and Energy LOss of Positrons and Electrons (PENELOPE)
16
,
17
and TOol for PArticle Simulation (TOPAS)
18
. The accuracy of the results from each code is directly related to the precision of internal particle transport, the interaction of radiation with matter, as well as the assignment of values used by the user
19
.
Thus, this study aimed to analyze the response of two Monte Carlo simulation packages: PENELOPE and TOPAS. The validation of the simulators was performed for two radiotherapy scenarios. For teletherapy, percentage depth doses were evaluated using electron and photon beams with both simulation packages. For the brachytherapy scenario, using the IR06-103Pd Palladium-103 model source, the behavior of the relative dose at depth was observed for both computational codes. The literature includes dosimetric analyses of brachytherapy performed in three materials: liquid water, solid water, and perspex
10
,
20
,
21
. Therefore, this study proposed to analyze dose deposition for both radiotherapy scenarios, investigating the importance of the material composing the phantom, varying between liquid water, solid water and perspex.
2. METHODS
2.1 Design
The study employed a comparative design between Monte Carlo simulation results and experimental and literature values. The analysis of this original study was based on quantitative approaches to dose deposition data for each of the dose scenarios addressed.
2.2 PENELOPE package
The PENELOPE simulation package is executed in FORTRAN subroutines and simulates the transport of electrons, positrons, and photons. Additionally, it has an available energy range from 1 keV to approximately 1 GeV
22
. The Monte Carlo method implemented in this algorithm allows for two-dimensional visualization of all defined geometries. The version used was 2014, and all simulations were performed on the cluster belonging to the Center for Technology and Information of Ribeirão Preto (Ceti-RP) at the University of São Paulo. Each core has 16 gigabytes of memory per node and an Intel i7 processor.
In this code, the transport of photons occurs sequentially, simulating interactions one after the other. These events are controlled by four user-defined parameters: C1, which corresponds to the average angular deflection produced by a hard collision, resulting in energy loss or a change in the particle’s direction, consequently leading to higher computational costs; C2, which represents the value of the maximum fractional energy loss dissipated in a hard collision; WCC, which symbolizes the maximum energy lost in hard collisions; and finally, WCR, which is the maximum energy lost by a charged particle in significant Bremsstrahlung emissions
22
,
23
.
2.3 TOPAS simulation package
The TOPAS simulation package operates using the GEANT4 algorithm tool of the Monte Carlo simulation code. It was developed with a more intuitive interface, allowing for three-dimensional visualization of geometries. This code enables the simulation of protons
24
, in addition to the particles also transported by PENELOPE. TOPAS was developed for high-energy physics, reaching radiobiological and clinical levels
18
, thus varying its available energy range from 1 eV to values close to TeV. The version used was 3.5, and all simulations were performed on a personal computer. For this code, the physics resource list of PENELOPE embedded in GEANT4 was loaded.
2.4 Simulation scenarios
The simulations were modeled using a cubic simulation phantom with sides of 30 cm, divided into 101 pixels in each of the three Cartesian axis directions. The filling of this phantom was varied among three different materials: liquid and solid water, and perspex. The composition of the latter two materials was configured according to Rodríguez et al., en 2005, and Saidi et al., en 2011
20
,
21
. The weight fractions (W) and densities (ρ) are listed in table 1. The number of primary particles simulated was kept constant at 5x10(8).
Table 1. Weight fractions of each constituent and densities of the materials of the phantom
| Material |
W H |
W ° |
W c |
W N |
W Ca |
W Cl |
ρ(g/cm3) |
| Liquid water |
66,00% |
34,00% |
- |
- |
- |
- |
01,00 |
| Solid water* |
08,09% |
19,84% |
67,22% |
02,40% |
02,32% |
00,13% |
01,01 |
| Perspex** |
08,00% |
32,00% |
60,00% |
- |
- |
- |
01,19 |
*Rodríguez et al., en 2005
20
; ** Saidi et al., en 2011
21
.
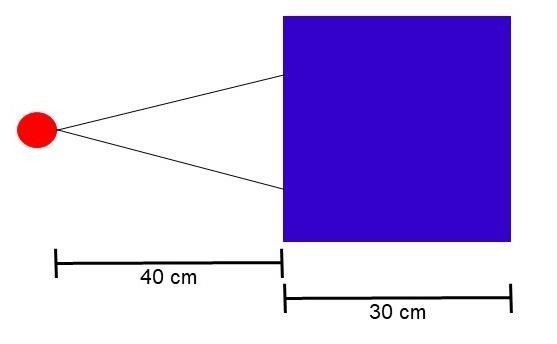
In the teletherapy scenario, the source was positioned 40 cm from the surface of the phantom, with a square irradiation field of 5 cm per side. The irradiation scheme is shown in Figure 1a. Photon and electron beams, both of 6 MeV, were analyzed. The values of the condensation parameters (C1 and C2) were set at 0.1; WCC and WCR were defined as 1x103 and 1x104, respectively, and the absorption energy for electrons, photons, and positrons (EABS) was set at 1x104 eV and 2x105 eV.
In the brachytherapy scenario, the simulated source was Palladium-103, model IR06-103Pd, according to Saidi & Sadeghi (2019)
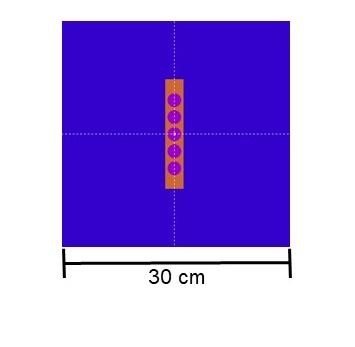
25
and it was modeled at the center of the phantom, as shown in Figure 1b. These simulations utilized the 8 main photon emission lines ranging from 20.07 keV to 497.1 keV. The values of C1 and C2 were both set to 0.3 to optimize simulation time, while WCC and WCR were defined as 5x103, and EABS was set at 5 keV.
Figure 1
Simulation scenarios: a) teletherapy, b) brachytherapy
a)
b)
3. RESULTS AND DISCUSSION
In this study, two radiation treatment scenarios, teletherapy and brachytherapy, were analyzed through simulation. For the former, percentage depth dose was evaluated while for the latter, relative dose was observed.
3.1 Teletherapy scenario
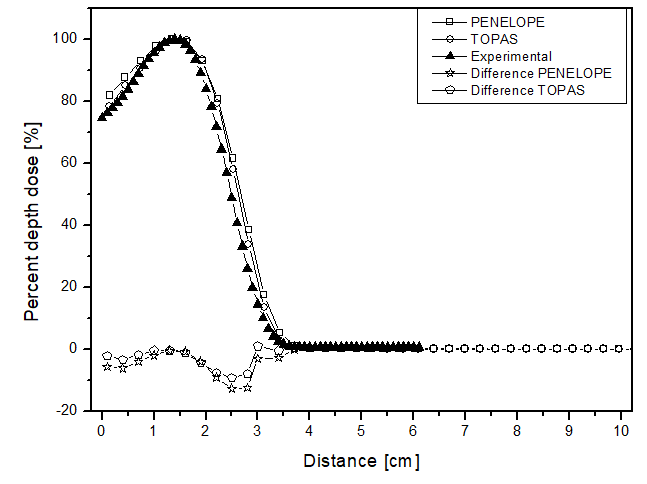
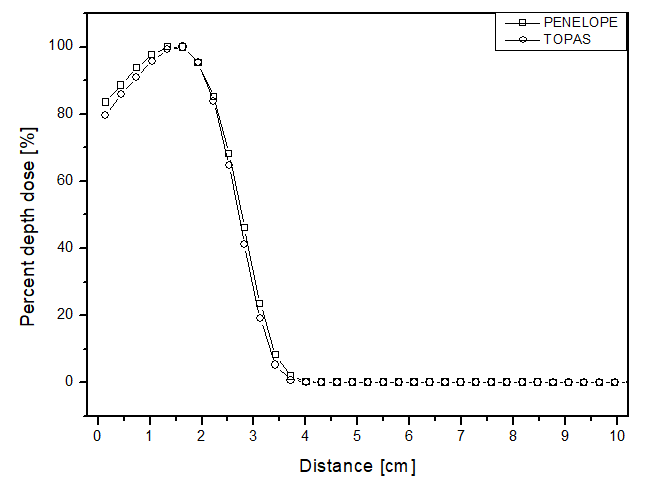
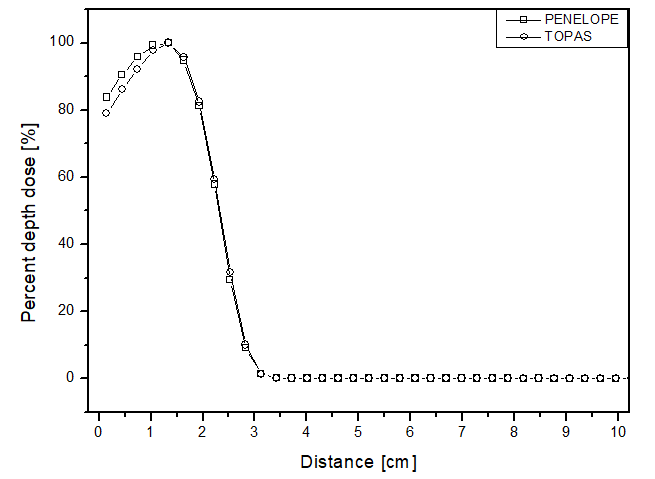
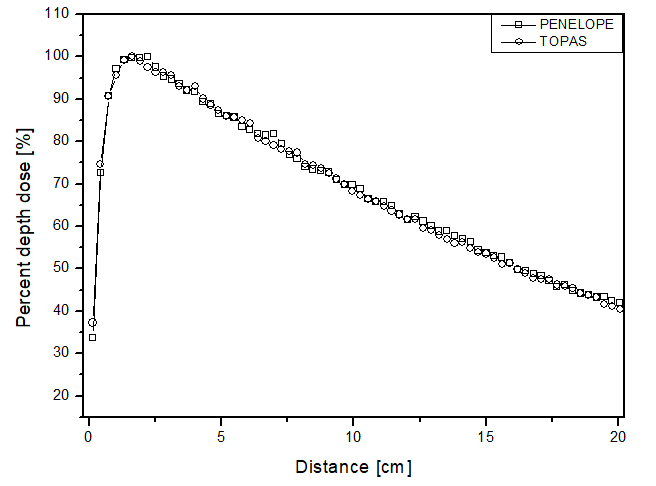
The scenario allowed for the analysis of two different beams. Figures 2 and 3 present the behavior for the electron and photon beams, respectively. Analyzing the graphs in figure 2, demonstrates a steep decline in performance up to approximately 4 cm distance for all materials in both cases. The behavior of the simulators aligns with experimental expectations, as the experimental dose deposition curve in figure 2a corresponds to the simulated curves. The largest point difference between simulations in liquid water compared to the experimental was 12 p.p with the PENELOPE Monte Carlo. However, after 5 cm of depth, the dose captured per pixel is approximately zero in both simulators. The largest variation between the curves of each simulator in each of the materials filling the phantom was also compared. PENELOPE showed the greatest difference compared to TOPAS, 4 p.p, when filled with liquid water. Among all scenarios developed for the electron beam, the maximum uncertainty found was less than 4.5%.
Figure 2
Percentage depth dose as a function of distance for the teletherapy scenario with electrons, with phantom filled with: a) liquid water, b) solid water y c) perspex
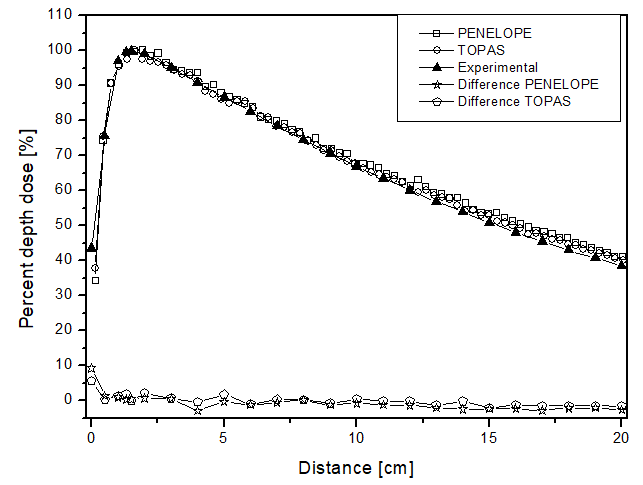
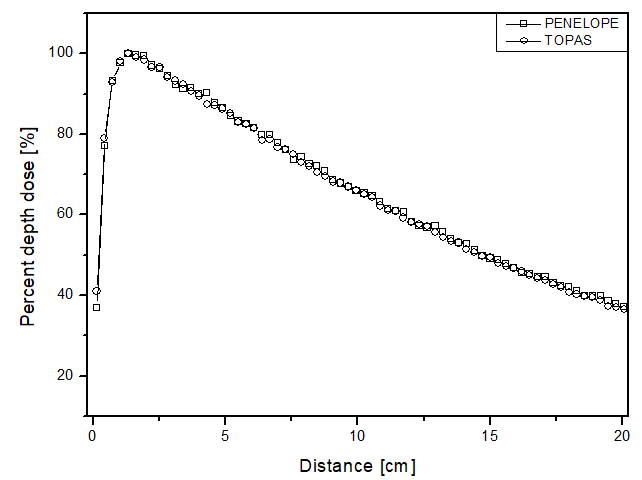
Unlike the electron beam, for the photon beam, the dose is found throughout the 10 cm of analysis. The behavior of the curves is similar for both computational codes, as the largest difference found was 4 p.p, which was higher for the TOPAS simulation package when the phantom was filled with perspex. The highest uncertainty found in the simulations for the photon beam was approximately 4.5%. In the comparative analysis with the experimental data, once again, the behavior of the dose deposition from the two simulators could be validated. This is because the largest difference found was 9 p.p, at 0.14 cm distance, with PENELOPE being the most distinct from the experimental data. These data are shown in figure 3a.
Figure 3
Percentage depth dose as a function of distance for the teletherapy scenario with photons and phantom filled: a) liquid water; b) solid water y c) perspex
3.2 Brachytherapy scenario
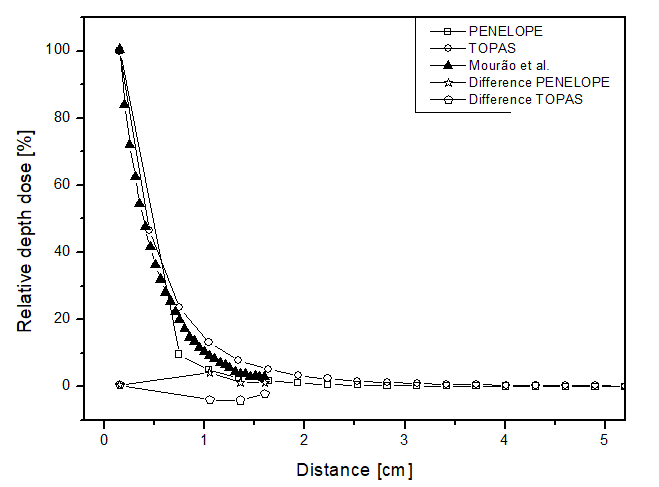
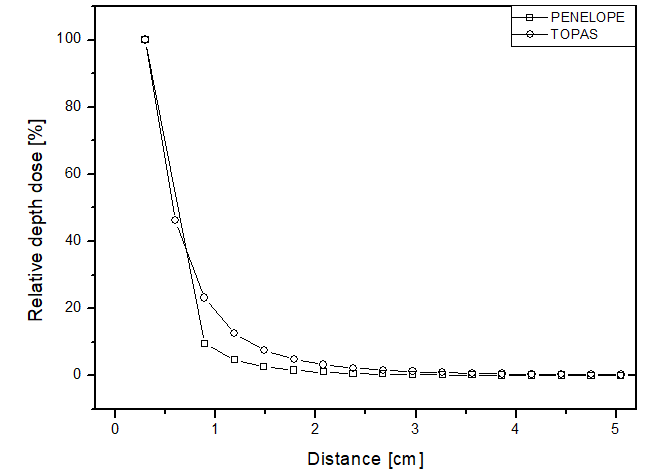
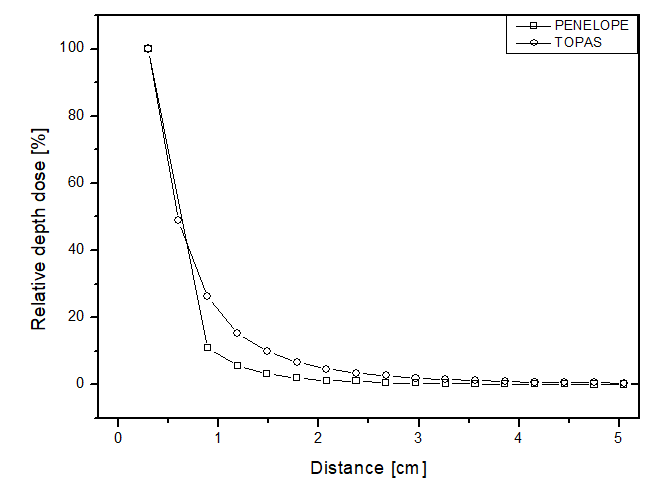
In this scenario, the comparison between the simulators was done through relative dose along the radial direction from the source for each of the materials. Figure 4 presents the obtained results, showing similar behaviors between the two simulators, but the point dose deposition up to 2 cm distance differed by up to 15 p.p between the curves for all materials. Figure 4a shows the dose curve obtained by Mourão & Campos, en 2010
26
, where the largest point difference was approximately 4 p.p for both simulators. Thus, as expected for a brachytherapy radiation source, the dose deposition obtained in the simulations was rapid, becoming approximately zero after 3 cm distance. This confirms the difficulty of performing dosimetry experimentally, considering the challenge of placing a dosimeter at such a distance from a source to ensure adequate detection. The simulators achieved a maximum relative uncertainty below 5% for all materials, thus meeting the AAPM recommendations.
Figure 4
Percentage depth dose as a function of distance for the brachytherapy scenario, with phantom filled with: a) liquid water; b) solid water; c) perspex.
Thus, the results presented for both scenarios are in agreement with what was expected, clinically and in the literature. Thus, the two computational Monte Carlo methods evaluated can be considered promising tools for performing dosimetry for brachytherapy. In addition, the agreement with the experimental results allows validating the codes as instruments for more advanced simulations that assist in predicting dose deposition in patients for new treatment techniques that can be studied.
4. CONCLUSION
In this study, depth dose profiles were analyzed for two types of teletherapy beams. In both cases, the behavior was similar to that expected from the literature, as the electron beam showed a steeper decline than the photon beam. Additionally, the electron beam exhibited lower penetrability along the phantom, similar to what occurs clinically, considering that electron beams are used for superficial treatments such as skin therapy.
The brachytherapy scenario also yielded satisfactory results for the comparison of the simulators. The curves for this type of source showed similar responses, with a point variation of 4 p.p near 1 cm. Furthermore, the behavior of the curves is analogous to the literature, with low uncertainty obtained for both computational codes. Thus, the analysis presented in this study demonstrates that the PENELOPE and TOPAS simulation packages exhibit high agreement in the results for teletherapy and brachytherapy scenarios.
Moreover, the simulation systems responded to the different compositions of the phantoms, showing variation of 4 p.p and 15 p.p for teletherapy and brachytherapy, respectively. Therefore, the studied simulation codes emerge as promising tools for dosimetry applications.
Acknowledgements
The authors would like to thank Center for Technology and Information of Ribeirão Preto (Ceti-RP) and Professor Alexandre Bonatto, from the Beam Physics Group (GFF) at University of Health Sciences of Porto Alegre (UFCSPA), for the computational resources made available to carry out the simulations, the latter being acquired with the help of National Council for Scientific and Technological Development (CNPq) (Universal Call 427273/2016- 1 and 405143/2021-4) and Rio Grande do Sul State Research Support Foundation (FAPERGS) (PqG 21/2551-0002027-0).