Introducción
Radiotherapy has been applied since the early 1950s
1
, and over the years, there has been significant advancement in the technologies and techniques of daily procedures, making the Treatment Planning System (TPS) more complex and patient-specific. Therefore, as TPS complexities increase, Quality Assurance (QA) methods must continuously evolve to ensure the proper functioning of the radiation dose delivery system
2
. Innovations in the field have led to the development of Intensity-Modulated Radiation Therapy (IMRT), which involves the movement of a collimator composed of multiple leaves to modulate the intensity of the radiation beam. With advancements, the technique has been refined into Volumetric Modulated Arc Therapy (VMAT), where the intensity of the dose rate beam is modulated with the gantry's movement. Another technique used is dynamic arc radiotherapy, which can shape the irradiation dose of a treatment, where the beam opening is continuously altered, and the leaves are dynamically adjusted to the target's shape through one or more rotations of the clinical linear accelerator's (LINAC) gantry. However, the dose rate and gantry speed have fixed values. Dynamic arc therapy can potentially ensure better treatment coverage, preserve normal tissue, and reduce dose delivery times
3
.
Proper treatment administration must be ensured through dosimetric analysis, which includes treatment plan verification through dose delivery and distribution measurements. The success or failure of a radiation treatment, as recommended by the International Commission on Radiation Units and Measurements, depends on the percentage difference between the absorbed dose at a reference point in the tumor and the prescribed dose for the same point. Dosimetric analysis precision should be within ± 5%
4
5
of the difference between both.
Possible sources of errors in radiotherapy include various factors, not limited to errors in the location of the organ to be treated, patient immobilization and positioning, and the calibration of the LINAC and its devices. These errors compromise treatment success and must be considered within the quality assurance program of the treatment through a rigorous periodic verification called patient-specific quality control
6
.
In order to minimize this degree of uncertainty, various specialized organizations recommend quality assurance programs. It is extremely important to perform patient-specific quality control (QC)
7
to ensure that the delivered dose distribution matches the dose prescribed by the radiation oncologist. Thus, the medical physicist is responsible for the QC and must create a methodology that allows testing, according to the resources available at their institution. For dosimetric analysis of point measurements, approval is ensured within the measurement error margin.
The gamma analysis is a method generally used to verify whether the planned dose distribution is equivalent to the one that would be delivered to the patient; for this, a comparison is made between the planned plane and an experimentally obtained distribution. The gamma index evaluates the dose difference and the distance to agreement (DTA) between two dose distributions
8-
11
.
The Task Group 218 (TG-218) of the American Association of Physicists in Medicine introduced more advanced concepts on tolerance limits and the methodologies used for patient-specific QA, recommending criteria of a 3% dose difference and 2mm DTA (3%/2mm), which are commonly used in clinical dosimetry
7
.
This study aimed to implement a quality control process for radiotherapy with dynamic arc technique in the LINAC CX, using the ArcCheck™ dose verification detector (Sun Nuclear Corp., FL), for clinical cases of patients already treated at a radiotherapy center in Brazil.
2. METHODS
2.1 Study Design
This study used an applied experimental design for patient-specific quality control, in which the performance of the ArcCHECK system was evaluated for patients treated with radiotherapy.
2.2Patient Selection
Fifty patients with different types of carcinomas were selected. They received treatment using the dynamic arc technique with the Varian CX LINAC, for irradiation fields larger than 5 x 5 cm². Of these patients, 17 were treated with 6 MV energy and thirty-three with 10 MV energy.
2.3 Materials and Equipment
Following the TG-119 recommendations, a 15 cm-high phantom, composed of flat solid water plates measuring 30 x 30 cm², was used, as shown in Figure 1.
The dosimetric systems used included a FC65-P Farmer cylindrical ionization chamber with a sensitive volume of 0.6 cm³. Additionally, an ArcCHECK™ detector (Sun Nuclear Corp., FL) was employed, which consists of a cylindrical water-equivalent phantom with an array of 1386 diode detectors arranged in a helical pattern to measure dose distributions. The LINAC used was a Clinac CX (Varian Medical Systems; Palo Alto, California, USA), operating with photon beams of 6 MV and 10 MV. The system is capable of producing shaped fields, as well as dynamic therapy involving gantry rotation, commonly known as dynamic arc.
The computerized treatment planning system (TPS) used was Eclipse version 15.5 (Varian Medical Systems, Palo Alto, CA, USA).
Figure 1
Positioning of the ionization chamber in the solid water phantom
2.4 Point Verification with the Ionization Chamber
Using the ionization chamber, it was possible to obtain dose values at the isocenter of the treatment plans, which were reproduced from the plans in the TPS Eclipse. The doses measured with the ionization chamber and the doses calculated by the TPS were compared for the plans with energies of 6 MV and 10 MV.
2.5 Dose Delivery and Verification
Parameters such as energy, field size, arcs, and other data from the treatment plans of each selected patient were obtained through the TPS.
Using the phantom shown in Figure 1, measurements were taken with an ionization chamber for point dosimetry
12-
at the isocenter of each treatment plan, and then compared with the data obtained from the TPS.
For the dose distributions, the parameters of the treatment plans were exported and experimentally simulated by the ArcCheck diode array. In conjunction with ArcCheck, the SNC Patient™ software projects the measurement onto the cylindrical surface into a plane and displays it similarly to a flat (2D) matrix, which can be reconstructed into a three-dimensional matrix
13-
15
.
Figure 2
PPositioning of the ArcCheck for the reproduction of a dynamic arc planning
2.6 Gamma Index Evaluation
For comparison of the planned and measured dose distributions, TG 218 recommends a gamma index criterion of 3%/2 mm with a universal tolerance of 95%, while the code of practice from the Netherlands Commission on Radiation Dosimetry suggests a criterion of at least 3%/3 mm with the same tolerance for all anatomical sites. Most institutions use the gamma index as the main criterion for plan approval, and it can also be used to approve patient-specific quality control
16
. In this evaluation study, there are dose distributions in the central plane of each treatment for each patient, respectively, for the energies of dynamic arc treatments with 6 MV and 10 MV photons.
3. RESULTS
3.1 IONIZATION CHAMBER DATA
The point dose values measured by the ionization chamber were calculated and compared with the values obtained with the TPS Eclipse. The doses measured by the ionization chamber and those calculated by the TPS for 6 MV and 10 MV energies are presented in Table 1 and Table 2, respectively.
The percentage variation in the difference between the calculated point doses ranged from -2.85% to 1.03% for 6 MV energy and from -3.68% to 2.04% for 10 MV energy. The average dose difference at the isocenter was -0.96% and -1.34% for 6 MV and 10 MV, as shown in Table 1 and Table 2, respectively.
Table 1.
Point dose measured and calculated at the isocenter using the ionization chamber; evaluation of dose distributions with gamma index for 3%/3 mm and 3%/2 mm for patients treated with a 6 MV beam.
| Patient No. |
Anatomical Region |
Ionization Chamber (cGy) |
TPS (cGy) |
Dose Difference (%) |
Gamma Index (3%/3mm) |
Gamma Index (3%/2mm) |
| 1 |
Flank |
372.62 |
374.20 |
-0.42% |
100.00% |
99.90% |
| 2 |
Esophagus |
237.72 |
239.40 |
-0.70% |
100.00% |
99.70% |
| 3 |
Lung |
217.62 |
222.30 |
-2.10% |
99.90% |
99.80% |
| 4 |
Esophagus |
192.77 |
195.80 |
-1.55% |
99.90% |
99.80% |
| 5 |
Esophagus |
220.67 |
225.30 |
-2.06% |
100.00% |
99.90% |
| 6 |
Bone MTS* |
257.47 |
262.10 |
-1.77% |
100.00% |
99.80% |
| 7 |
Esophagus |
179.18 |
183.40 |
-2.30% |
99.70% |
99.40% |
| 8 |
Shoulder MTS* |
300.09 |
308.90 |
-2.85% |
100.00% |
99.80% |
| 9 |
Breast |
311.03 |
312.90 |
-0.60% |
100.00% |
100.00% |
| 10 |
Esophagus |
291.71 |
290.30 |
0.48% |
100.00% |
99.80% |
| 11 |
Esophagus |
189.64 |
188.20 |
0.77% |
99.80% |
99.70% |
| 12 |
Scape MTS* |
304.13 |
305.40 |
-0.42% |
98.90% |
98.50% |
| 13 |
Esophagus |
200.29 |
202.50 |
-1.09% |
|
|
| 14 |
Esophagus |
225.32 |
227.50 |
-0.96% |
99.90% |
99.60% |
| 15 |
Right foot |
329.80 |
333.70 |
-1.17% |
100.00% |
100.00% |
| 16 |
Sternum MTS* |
291.56 |
288.60 |
1.03% |
100.00% |
99.70% |
| 17 |
Lung |
221.33 |
219.70 |
0.74% |
99.90% |
99.70% |
*MTS: Metastasis
Table 2.
Point dose measured and calculated at the isocenter using the ionization chamber; evaluation of dose distributions with gamma index for 3%/3mm and 3%/2mm for patients treated with a 10 MV beam
| Patient No. |
Anatomical Region |
Ionization Chamber (cGy) |
TPS (cGy) |
Dose Difference (%) |
Gamma Index (3%/3mm) |
Gamma Index (3%/2mm) |
| 1 |
Left Lung |
207.80 |
212.04 |
2.04% |
94.50% |
93.00% |
| 2 |
SVC |
339.70 |
334.75 |
-1.46% |
99.90% |
99.70% |
| 3 |
Left Rib MTS* |
346.60 |
352.68 |
1.75% |
99.79% |
99.10% |
| 4 |
Esophagus |
228.80 |
225.02 |
-1.65% |
99.80% |
99.70% |
| 5 |
Right Lung |
266.10 |
264.75 |
-0.51% |
99.60% |
98.90% |
| 6 |
T3 MTS* |
354.30 |
345.83 |
-2.39% |
100.00% |
99.90% |
| 7 |
Right Rib |
278.80 |
275.00 |
-1.36% |
100.00% |
98.50% |
| 8 |
L3-L5 MTS* |
500.50 |
493.08 |
-1.48% |
100.00% |
100.00% |
| 9 |
T7 MTS* |
324.60 |
313.48 |
-3.43% |
100.00% |
99.90% |
| 10 |
C1 MTS* |
293.20 |
288.47 |
-1.61% |
100.00% |
100.00% |
| 12 |
Inguinal MTS* |
327.30 |
324.84 |
-0.75% |
100.00% |
99.80% |
| 13 |
Left Hip |
326.70 |
326.70 |
0.00% |
100.00% |
99.90% |
| 14 |
Abdomen MTS* |
343.40 |
343.09 |
-0.09% |
99.80% |
99.70% |
| 15 |
Mediastinum |
325.20 |
325.99 |
0.24% |
100.00% |
100.00% |
| 16 |
Right Adrenal |
330.70 |
330.15 |
-0.16% |
99.70% |
99.30% |
| 17 |
Esophagus |
176.50 |
174.13 |
-1.34% |
100.00% |
99.90% |
| 18 |
Esophagus |
198.30 |
196.46 |
-0.93% |
100.00% |
100.00% |
| 19 |
Right Lung |
238.30 |
234.05 |
-1.78% |
98.80% |
95.70% |
| 20 |
Left Lung |
215.10 |
212.37 |
-1.27% |
99.60% |
97.80% |
| 21 |
Right Femur MTS* |
294.20 |
292.25 |
-0.66% |
99.80% |
99.70% |
| 22 |
Stomach |
320.90 |
319.90 |
-0.31% |
98.60% |
97.10% |
| 23 |
Bone MTS* |
312.90 |
310.67 |
-0.71% |
99.60% |
98.90% |
| 24 |
Esophagus |
204.20 |
196.70 |
-3.68% |
99.40% |
99.00% |
| 25 |
Left Breast |
294.40 |
286.40 |
-2.72% |
100.00% |
100.00% |
| 26 |
Spine MTS* |
318.10 |
312.72 |
-1.69% |
100.00% |
99.80% |
| 27 |
Bladder |
358.70 |
346.27 |
-3.47% |
100.00% |
99.60% |
| 28 |
Right Breast |
270.70 |
263.20 |
-2.77% |
99.90% |
99.60% |
| 29 |
Esophagus |
199.60 |
195.23 |
-2.19% |
99.90% |
99.90% |
| 30 |
Right Inguinal |
287.70 |
285.33 |
-0.83% |
99.80% |
98.70% |
| 31 |
Esophagus |
218.40 |
214.96 |
-1.58% |
100.00% |
100.00% |
| 32 |
T12 + Lumbar |
383.80 |
378.84 |
-1.29% |
100.00% |
99.90% |
| 33 |
Right Bone MTS* |
378.80 |
372.93 |
-1.55% |
99.70% |
99.50% |
*MTS: Metastasis
3.2 Data Obtained with ArcCheck
The recommendations of TG-218 were followed using the 3%/2mm criterion and the current routine service criterion of 3%/3mm. For patients treated with 6 MV energy, the number of points that passed the gamma criterion ranged from 99.70% to 100.0% and from 99.50% to 100.0% for 3%/3mm and 3%/2mm, respectively (Table 1), with an average of 99.87% and 99.67%. For patients treated with 10 MV energy, the results ranged from 94.50% to 100.0% and from 93.00% to 100.0% for 3%/3mm and 3%/2mm, with an average of 99.60% and 98.90%, as shown in Table 2.
4. DISCUSSION
The reference dosimeter for quality control in radiotherapy is the ionization chamber, as recommended by the International Atomic Energy Agency (IAEA) TRS-398 protocol. It is also suggested that the ionization chamber must be properly calibrated, as it is considered one of the most reliable methods for absolute dose measurements
17
. As shown in Tables 1 and 2, with percentage differences smaller than 4% for both evaluated energies across all treatments, when comparing the data obtained with the ionization chamber and the TPS. However, the ionization chamber performs a single point measurement (1D), which may not be an adequate verification for radiotherapy techniques using dynamic arc. In light of this situation, the ArcCheck detector was implemented to evaluate dose delivery accuracy in three dimensions. Thus, dose matrices allow for three-dimensional dosimetry, making it a competent means to ensure patient-specific quality control
18
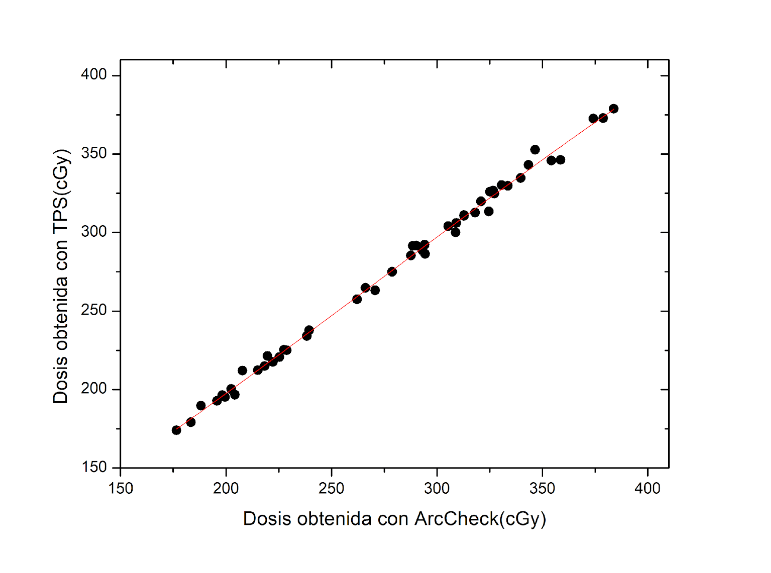
. In this study, the dose distributions in the central plane of each treatment were compared for each patient, with the dose distributions obtained from ArcCheck and the TPS. From the percentage difference between the dose distributions for each patient, an average dose was obtained for all cases, resulting in a mean percentage difference of -0.58% ± 0.04. To visualize the relationship between the two variables, the concordance correlation coefficient was calculated between the dose measured by ArcCheck and the TPS, resulting in 0.99, as shown in Figure 3.
Figure 3
Trendline of the Dose Calculated by TPS versus the Dose Measured by ArcCheck
In relation to the gamma index, each dose point was measured and compared with the calculated dose, seeking an analogous dose within the defined criteria of 3%/3mm and 3%/2mm. The results obtained were consistent with TG-218 and TG-119 protocols, with an approval average higher than 98.0% for both energies, demonstrating excellent concordance. Through the gamma index, which is a useful tool for dosimetric verification, the TPS planning was compared with the data obtained with ArcCheck, showing that it is an adequate metric for evaluating dynamic arc plans. This study highlights the importance of developing a specific protocol for each institution, as the gamma index depends on treatment planning and setup, considering the type of dosimeter, detector resolution, TPS algorithm, linear accelerator configuration, and the clinical judgment of dose tolerance level, which also influence the outcome, as shown when using the evaluated criteria (3%/3mm and 3%/2mm).
5. CONCLUSIONS
This study demonstrates the efficacy and accuracy of the ArcCheck detector in implementing a patient-specific quality control technique for patients treated with the dynamic arc technique. The evaluation of 50 patients treated with 6 MV and 10 MV energies revealed a minimal average difference in the doses measured at the isocenter, with values of -0.96% and -1.34%, respectively. Additionally, the gamma analysis showed a high approval rate, above 98.0% for both energies, underscoring the reliability of ArcCheck in validating dose distributions. The consistency of these results with TG-119 and TG-218 protocols strongly supports the adoption of ArcCheck as an essential tool for quality control in specific radiotherapy treatments, ensuring the safe and precise delivery of ionizing radiation.