ORIGINAL ARTICLE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2019 - Universidad Ricardo PalmaDOI 10.25176/RFMH.v20i1.2447
MICROSCOPIC FINDINGS OF LUNG TISSUE AND RESPIRATORY MUSCLES IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD)
HALLAZGOS MICROSCÓPICOS DEL TEJIDO PULMONAR Y LOS MÚSCULOS RESPIRATORIOS EN LA ENFERMEDAD PULMONAR OBSTRUCTIVA CRÓNICA (EPOC)
Tales Lyra de Oliveira1,2,a,
Tácio Nertan Cartaxo de Souza3,b,
Byron Martins Lucena4,c,
Fernanda Mayara Santos Santana5,d,
Lysien Ivania Zambrano6,e,
Ana Rose de Carvalho Araujo7,f,
Ana Lúcia de Gusmão Freire8,g
1 Faculty of Medicine, Municipal University of São Caetano del Sur. São Paulo, Brasil.
2 Hypertension Unit, Heart Institute, Faculty of Medicine, University of Sao Paulo. São Paulo, Brasil.
3 Institute of Biological and Health Sciences, Federal University of Alagoas. Maceió, Brasil.
4 Municipality of Maceió. Maceió, Brasil.
5 Faculty of Medicine, Federal University of Alagoas. Maceió, Brasil.
6 Department of Morphological Sciences, Faculty of Medical Sciences. National Autonomous University of Honduras,Tegucigalpa, Honduras.
7 Death Verification Service, Alagoas State University of Health Sciences. Maceió, Brasil.
8 Faculty of Physiotherapy, Alagoas State University of Health Sciences. Maceió, Brasil.
a Physiotherapist, Doctor of Sciences
b Physiotherapist, Master of Health Sciences
c Physiotherapist
d Physiotherapist, Medical Student
e Surgeon, Master of Science
f Pathologist
g Physiotherapist, Master in Physical Therapy
ABSTRACT
Objective: To evaluate the histopathological changes present in the lungs and respiratory muscles (diaphragm and ECOM) of the corpses with a diagnosis of COPD.
Methods: This is a blind and descriptive study with analysis of histological images of biopsy. The history of smoking associated with the presence of pulmonary anthracosis, septal thickening and emphysematous bullae included the inclusion criteria of the study. The sample consisted of lung, diaphragm and ECOM biopsies. The study was performed by optical microscopic analysis of histological sheets obtained from 36 bodies with COPD. The histopathological diagnosis was made by a pathologist who did not know the objectives of the study.
Results: In the diaphragm, there was the presence of interposed adipose tissue, muscular atrophy, removal of muscle fibers and fibrosis. In ECOM, the elimination of muscle fibers, muscular atrophy, interposed adipose tissue, muscle hypertrophy and thickening of the tendons were also evident.
Conclusion: The changes found in the diaphragm and ECOM muscle biopsies of the bodies with COPD were evidenced as a mechanism of compensation and / or dysfunction of the respiratory system due to biomechanical alterations promoted by the disease.
Keywords: Chronic obstructive pulmonary disease, Striated muscle, Anthracosis, Tobacco Use Disorder, Autopsy. (Source: MeSH MEDLINE)
RESUMEN
Objetivo: Evaluar los cambios histopatológicos presentes en los pulmones, diafragma y y esternocleidomastoideo (ECOM) en cadáveres con diagnóstico de Enfermedad Pulmonar Obstructiva Crónica (EPOC).
Métodos: Este es un estudio ciego y descriptivo con análisis de imágenes histológicas de biopsia. El historial de tabaquismo asociado con la presencia de antracosis pulmonar, engrosamiento septal y bullas enfisematosas comprendió los criterios de inclusión del estudio. La muestra consistió en biopsias de pulmón, diafragma y ECOM. El estudio se realizó mediante análisis microscópico óptico de láminas histológicas obtenidas de 36 cadáveres con EPOC. El diagnóstico histopatológico fue realizado por un patólogo que desconocía los objetivos del estudio.
Resultados: En el diafragma, hubo la presencia de tejido adiposo interpuesto, atrofia muscular, eliminación de las fibras musculares y fibrosis. En el ECOM, también se evidenció la eliminación de las fibras musculares, la atrofia muscular, el tejido adiposo interpuesto, la hipertrofia muscular y el engrosamiento de los tendones.
Conclusión: Los cambios encontrados en las biopsias musculares de diafragma y ECOM de los cadáveres con EPOC se evidenciaron como un mecanismo de compensación y/o disfunción del sistema respiratorio debido a alteraciones biomecánicas promovidas por la enfermedad.
Palabras Clave: Enfermedad pulmonar obstructiva crónica, Músculo estriado, Antracosis, Tabaquismo, Autopsia. (Fuente DeCS BIREME)
More than 3 million people died of COPD in 2012, representing 6% of all deaths worldwide(1-2). In Brazil, COPD is considered the fourth cause of death, affecting more than 6 million people and victimizing approximately 30,000 people per year(3). Data indicate that 2/3 of all male and 1/7 of all female autopsies show signs of pulmonary emphysema(4).PLATINO study, conducted in five metropolitan regions of Latin America, showed that the prevalence of COPD in population over 40 years of age in São Paulo was 15.8%(5).
Chronic air flow limitation, characteristic of COPD, is predominantly caused by an association of small airways diseases (obstructive bronchiolitis) and destruction of lung parenchyma (pulmonary emphysema), which promotes a lung hyperinflation condition(6).
Chronic exposure to pathogens, including cigarette smoke, leads to chronic inflammation in lung parenchyma, leading to an imbalance between proteolytic and antiprotolytic enzymes(7). Therefore, any factor that increases the production or activity of proteases, especially elastases, and/or reduces or inhibits anti-elastases, promotes the destruction of pulmonary elastic structure. The most important anti-elastase is alpha-1-antitrypsin, and the main sources of elastase are neutrophils, which rise along with macrophages, T-lymphocytes and eosinophils in smokers(8). Alpha-1-antitrypsin, which acts to defend lung tissue against elastases, is inactive due to the action of oxidizing substances released by cigarette smoke(9). Therefore, pulmonary emphysema is the result of the destructive elastolytic effect in smokers with low anti-elastase activity. As elastase is expressed almost exclusively by neutrophils, smokers have more neutrophils in their bronchoalveolar lavage (BAL) than non-smokers, so neutrophils are considered the principal cause of pulmonary emphysema(7).
From these data, there is likely to be a complex inflammatory response in the emphysema lung. It is not known exactly how the various types of inflammatory cells contribute to the destruction of the alveolar walls, but reasonable speculation is that neutrophils, macrophages and eosinophils directly degrade the alveolar extracellular matrix by releasing proteinases, while T-lymphocytes influence cell recruitment. Inflammatory proteins that spread proteinases(10).
In COPD, the diaphragm acts against increased mechanical loads due to limited air flow and changes in chest conformation due to lung hyperinflation condition. It is suggested that this mechanical overload imposed on the diaphragm simulates resistance training (11). Strength and endurance characterize the muscle performance of the diaphragm. Therefore, the loss of these characteristics results in diaphragmatic muscle weakness and, consequently, its worse mechanical performance(12). Literature has consistently shown that people with severe COPD generate less maximum inspirational pressure and transdiaphragmatic pressure in voluntary maneuvers compared to people without the disease(13,14). Therefore, muscle strength is considered a limiting factor in diaphragm performance in patients with COPD. The scalene and sternocleidomastoid muscles are inspiratory muscles with similar actions on the chest wall, which generate a cranial displacement of the sternum and ribs. However, these muscles have different patterns of activity. Scalenes are inspiratory muscles activated in each ventilatory cycle, even during rest, while SCM muscles are accessory muscles of inspiration and do not contract during basal breathing, but do so only after reaching approximately 70% of inspirational capacity(15).
Based on these data, the aim of this study was to evaluate the histopathological changes present in the lungs and respiratory muscles (diaphragm and SCM muscles) of corpses affected by COPD.
METHODS
Design of the study
This is a descriptive study with findings of histologic biopsy images using a non-probabilistic sample. Samples were collected at the Death Verification Service of the Alagoas State University of Health Sciences (UNCISAL), from April to September 2012. The UNCISAL Ethics Committee approved Protocol 1796/2011 and those legally responsible for the autopsy bodies were informed of the procedures and signed the informed consent form attached to the general protocol of admission to the Death Verification Service. Initially, information on the body was collected from the Death Verification Service obituary form, completed by the legal guardian, which considered gender, age, race, profession, origin and presence of a smoking history, considered as the main criteria for inclusion in the study.
Thirty-six (36) corpses with histopathological diagnosis of COPD confirmed by the presence of pulmonary anthracosis, septal thickening and emphysema bullas were included in the study. Bodies without lobe or lung resulting from ablative surgery or congenital malformation were excluded from the study, with a significant presence of pleural adhesions that suffered parenchymal lesions by the necropsy procedure and corpses with comorbidities such as neuromuscular disease and structural bone malformation of the rib cage. Thus, eight corpses were excluded from the study, three because they did not present the histopathological findings expected for COPD; four because they had intense pleural adhesions and one because it was diagnosed with neuromuscular disease (Parkinson’s disease).
Lung and muscle biopsies
The material for analysis was collected using the standard necropsy procedure, which performed a longitudinal section from the manubrium region to the umbilical scar to access the thoracic and abdominal structures from which bilateral samples of the pulmonary apex were collected (Figura 1.A). SCM sections were performed bilaterally on the ventral side near its insertion into the sternum handlebar (Figura 1. B). While the diaphragm muscle was sectioned in its right lateral-costal region (Figura 1. C).
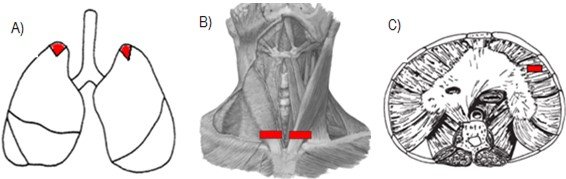
Figure 1. Sections for experimental protocol.
A) Section in pulmonary apex. B) Section in the muscular belly of the SCM muscle, bilaterally, near its insertion in the handlebar; C) Section in the right lateral-costal region of the diaphragm.
Histological processing
Five tissue fragments were obtained from each corpse to make a total of 288 histological plates, which allowed the identification and localization of histopathological changes, by means of two different stains. For histological visualization, tissues were fixed with 10% diluted formaldehyde, dehydrated with different ethanol concentrations, clarified with xilol and included in paraffin. The paraffin material was then cut to 5 µm in microtome thickness and the deparafinned sections were rehydrated and stained with hematoxylin-eosin (HE) for structural staining of all evaluated tissues and trichrome staining of Masson for fibrosis in muscle tissues. Therefore, it was necessary to make two histological markers for each muscle tissue, using for one sample the coloration by the HE method and the other by the trichrome of Masson. Pulmonary parenchyma was evaluated only by staining with HE. The histological plates were analyzed using conventional optical microscopy and the histopathological diagnosis of the biopsies was carried out by a Death Verification Service pathologist, who was unaware of the study’s objectives.
RESULTS
The socio-demographic profile of the corpses showed that males predominated (63.9%, n=23), mulatto (66.7%, n=24), urban-related profession (77.8%, n=28), residence in the capital (61.1%, n=22) and cause of death related to cardiopulmonary disease (80,6%, n=29, Table 1). The average smoking time of the sample was 31.3 ± 19.6 years.
TABLE 1. Sociodemographic profile of the biopsied sample of corpses with COPD.
| Biopsies (n=36) | |
| Gender | |
| Female | 36,1% (13) |
| Male | 63,9% (23) |
| Age group(años) | 67,2 ± 17,4 |
| Race | |
| White | 30,5% (11) |
| Mulatto | 66,7% (24) |
| Black | 2,8% (1) |
| Profession | |
| Rural zone | 22,2% (8) |
| Urban zone | 77,8% (28) |
| Origin place | |
| Capital | 61,1% (22) |
| Interior | 38,9% (14) |
| Time of tobacco use (Years) | 31,3 ± 19,6 |
| Death cause | |
| Cardiopulmonary diseases | 80,6% (29) |
| Other diseases | 19,4% (7) |
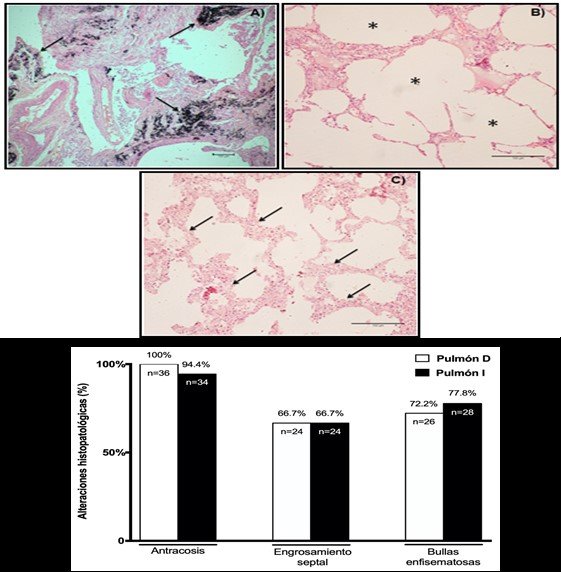
Figure 2.Microscopic visualization of lung biopsies and histopathological alterations.
Images of histological sections of lung tissue stained with hematoxylin-eosin (HE) in corpses with COPD. A) Pulmonary anthracosis (4x magnification). B) Emphysema bubbles. (10x magnification). C) Septal thickening (10x magnification). D) Representative graphic of results of right lung (R Lung) and left lung (L Lung) biopsies in corpses with COPD.
Regarding diaphragm biopsies, most relevant findings were the presence of interpolated adipose tissue (33.3%, n=12) and muscle atrophy (25%, n=9), and to a lesser extent, the distancing of muscle fibers (19.4%, n=7) and the installation of fibrosis (2.8%, n=1). The results of diaphragm biopsies can be seen in Figure 3. A, B, C, D y E, respectively.

Figure 3.Microscopic visualization of diaphragm muscle biopsies and histopathological alterations.
Images of histological cuts of diaphragm muscle stained with Masson’s trichrome technique on corpses with COPD: A) Muscle fibrosis (40x magnification). Images of histological plates of diaphragm stained with hematoxylin-eosin (HE) technique in corpses with COPD: B) Muscular atrophy (10x magnification). C) Interposed adipose tissue (20x magnification). D) Separated fibres (20x magnification). E) Representative graphic of the results of biopsies of diaphragm muscle in corpses with COPD.
SCM biopsies showed converging results in fibre distance (R SCM: 19.4%, n=7; L SCM: 16.7%, n=6) and muscle hypertrophy (R SCM: 13.9%, n=5; L SCM: 11.1%, n=4), and muscle atrophy to a lesser extent (R SCM and L: 11.1%, n=4), interposed adipose tissue (R SCM: 5.6%, n=2; L SCM: 8.3%, n=3) and tendon thickening (R SCM and L: 5.6%, n=2). The results of SCM biopsies can be seen in Figure 4. A, B, C, D y E, respectively.
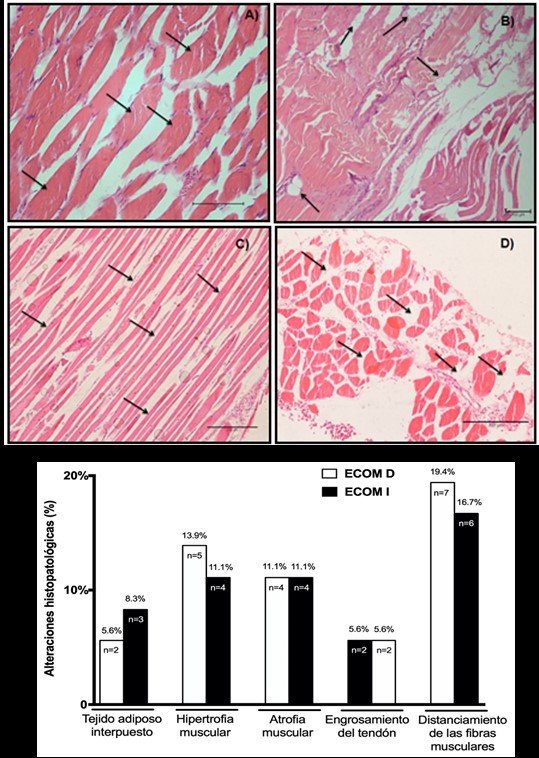
Figure 4.Microscopic visualization of biopsies of sternocleidomastoid muscles (SCM) and histopathological alterations.
Images of histological sections of the sternocleidomastoid muscles (SCM) stained with the hematoxylin-eosin technique (HE) in corpses with COPD: A) Fiber hypertrophy (20x magnification). B) Fatty tissue interposed between myofibrils (4x magnification). C) Muscular atrophy (10x magnification). D) Fibres far from SCM (10x magnification). E) Representative graphic of biopsy results between right (R SCM) and left sternocleidomastoid muscles L SCM) in corpses with COPD.
DISCUSSION
Anthracosis is a lesion characterized by the accumulation of charcoal dust in lungs and, with the consequent reaction of the tissue to its presence. Of all the exogenous factors that cause problems to the body, the most frequent is the carbon present as the main pollutant of the atmospheric air. Carbon is inhaled as particles small enough to reach the alveoli and is phagocyted by macrophages that can return its carbon charge to the interstitial lung tissue where it enters the lungs to be deposited preferably in the hiliar and mediastinal lymph nodes(16).
Therefore, smoker individuals exhibit a blackened lung pigmentation which, when viewed below the pleura, is arranged in lines surrounding the periphery of the pulmonary lobes. Evidence of anthracosis by contamination does not appear to be a predisposing factor for any lung disease. However, when associated with smoking, aggravates the harmful effects on the lungs, this has been demonstrated in studies with coal miners, and it was observed that among miners only smokers developed lung alterations sufficient to produce symptoms(17).
Emphysema blisters occur due to the irreversible decrease in the amount of elastic fibers in the alveolar septa due to the reduction of the renewal of the elastic tissue and its synthesis qualitatively defective corresponds to a subpleural or intraparenchymal alteration more than 1 cm in diameter and is surrounded by a fibrous wall consisting of visceral pleura, remains of alveolar septum, blood vessels and anthracosis, and its interior is full of air(18). In addition, histologically, septal thickening is explained in most cases by an intense inflammatory process that tends to heal, as well as by the exacerbation of reticulin and collagen fibers in the alveolar partitions(19).
Pulmonary hyperinflation represents one of the critical factors capable of altering the function of the respiratory muscles, especially when it induces a shortening of the diaphragm, placing it in a suboptimal position, reduces its relationship between length and tension and generates its mechanical disadvantage, observed in our study. In addition, diaphragmatic fibers may alter their mechanical arrangement leading to a reduced capacity to generate force(11). Therefore, if there were no adaptations in muscle length to chronic hyperinflation, the diaphragm would remain in a shortened state during breathing and its ability to contribute to inspirational pressure would be reduced(20).
In the present study, it was found that the majority of the individuals had structural changes in the diaphragm, in which muscular atrophy was the most frequent histopathological finding. Atrophy of the diaphragm may be justified by an increase in the rate of proteolysis in damaged or altered muscle proteins. Some authors have pointed out the possibility that the diaphragm and other respiratory muscles have significantly high degrees of muscle injury due to myonuclear apoptosis and/or oxidative stress due to overload(19).
The degradation of damaged proteins or target proteins that lead to the process of atrophy and shortening of muscle fiber is considered one of the first steps for the ubiquitination process through the proteasome. Literature data indicate that there is an increase in proteasome activity, indicated by activation of ubiquitin-proteasome pathway, in the diaphragm of patients with COPD(12). Therefore, these data suggest that permanent damage to diaphragm fibers may be related to inspirational overloads and/or changes in thoracic conformation, contributing to the muscle remodeling process.
Presence of interposed adipose tissue was found in a large number of muscle biopsies with diaphragm reported by our study which was endorsed by Conte et al(21) which indicate that the ageing of the skeletal striated muscle is a complex phenomenon, consisting of various physiological and morphological alterations, such as the replacement of muscle fibers with adipose and fibrotic tissue, as well as a decrease in protein synthesis, which causes a reduction in muscle strength and efficiency(21). Another predisposing factor for the interposition of adipose tissue is the presence of muscular atrophy found in the multifidial lumbar muscles in patients with leg pain evaluated by magnetic resonance imaging(22).
From this evidence, Kader et al. characterized as mild muscular atrophy the replacement of less than 10% of the multifidium muscle mass with adipose and fibrous tissue; as moderate muscular atrophy substitution between 10% and 50%; severe atrophy is the replacement of more than 50% of muscle tissue associated with atrophy of other paravertebral muscles.
Similarly, the present study found the presence of muscle atrophy in diaphragm biopsies, reinforcing the fact that muscle tissue can be replaced by adipose tissue, confirmed by histological images of adipocytes interposed between myofibrils. Elimination of myofibrils can be justified by the reduction of the cross-sectional area of the diaphragmatic fiber in the condition of severe COPD, which may represent a beneficial structural adaptation, facilitating oxygen transport and diffusion through capillaries in muscle fibers to meet the metabolic demand of tissue(12). Another study reported that messenger RNA levels in the diaphragm and endothelial vascular growth factor increase in patients with COPD, suggesting an improved angiogenesis condition(23). In this way, the detachment of fibers can occur as an adaptive mechanism of muscle tissue in the distribution of new capillaries.
Presence of fibrosis, connective scar tissue composed by collagen accumulation in the diaphragm, was another interesting finding. Mechanical overload can have significant detrimental effects on respiratory muscles and can induce injuries(12). Studies show that experimental models with high respiratory overloads showed lesions in the diaphragm(24,25). Tissue injury is followed by the activation of satellite cells that proliferate and differentiate into new muscle cells that contribute to the repair process with the formation of heavy myosin chains(25). However, some studies have suggested that the differentiation of satellite cells from the diaphragm into new muscle cells is affected in the COPD condition(14,20).
Therefore, due to the deterioration of the repair mechanism, replacement of muscular tissue by collagen occurs as an attempt to prevent further injury. Injuries and collagen accumulation lead to loss of the ability of the individual with COPD to generate strength. The reduction in the volume of muscle fibers in operation can be compensated by hypertrophy or increased muscle activation, however, because the diaphragm is mechanically disadvantaged, is incapable of hypertrophy, promoting in some cases the atrophy of their fibers(19). An orthopedic study has shown that areas of muscle overload can lead to inflammation of the tendon with degeneration and disorganization of collagen fibrils, a condition associated with thickening of the tendon(26).
Interestingly, the diaphragm of patients with moderate and severe COPD was considered three times more susceptible to the rupture of their sarcomeres when subjected to ventilation versus additional inspirational loads compared to patients without COPD(27). According to these results, data from a study of necropsy bodies with severe COPD revealed that an acute increase in chronic respiratory overload induces extensive diaphragmatic lesion and collagen accumulation(19).
With respect to SCM tissue alterations found in this study, it is suggested that the effect of pulmonary hyperinflation on inspiratory muscles, such as intercostal, scalene and SCM, leads to an increase in the diameter of the ribcage, which limits the biomechanical efficiency of these muscles(11). Changing the type of fiber that occurs in opposite directions in the diaphragm and other muscle groups has been shown to be one of the most common changes in the breathing muscles of people with COPD. Thus, while the change in the type of diaphragmatic fiber is associated with the gain of strength (type I muscle fibers)(28,29).
It is important to note that SCM hypertrophy was also observed in this study in a large number of biopsies. According to Hudson and his colleagues, COPD patients require additional recruitment of inspirational accessory muscles, especially SCM muscles, in an attempt to maintain adequate lung filling pressures, which suggests that SCM’s hypertrophy is a compensatory attempt to improve its exercise capacity and to generate inspirational force, since the diaphragm had a reduced capacity and was more resistant to fatigue(29).
Interestingly, some SCM biopsies showed muscle atrophy, which corroborates the literature data showing that aging favors the loss of motor neurons and, consequently, denervation of neuromuscular junction. These changes occur unevenly between different muscle groups, preferably affecting type II fibers that tend to be more present in SCM and cause some degree of muscle atrophy(30).
CONCLUSION
Data from this study indicate that there are structural histological changes in the diaphragm and SCM respiratory muscles of COPD-affected corpses, presumably due to the condition of pulmonary hyperinflation and the ensuing biomechanical changes in the ribcage. However, further studies are needed to clarify whether such changes are a compensatory mechanism to optimize pulmonary ventilation or a consequence of structural systemic dysfunction.
Authorship contributions: The authors participated
in the conception, drafting and final approval of the
Original article, plus ERQ in data collection.
Conflict of interest: Los autores declaran no tener conflictos de interés.
Financing: self-financed
Received: 30 October 2019
Approved: 13 December 2019
Correspondence: Tales Lyra de Oliveira
Address: Universidade Municipal de São Caetano do Sul, Faculdade de Medicina, Campus Bela Vista, Rua Treze de Maio, 681, Bela Vista, São Paulo - SP, CEP 01327-000
Phone number: +55 11 2730-0030
E-mail: tales.oliveira@prof.uscs.edu.br
REFERENCES
