CASE REPORT
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2021 - Universidad Ricardo Palma10.25176/RFMH.v23i1.4992
ACUTE PNEUMONITIS AFTER SUBCUTANEUS INJECTION OF BIO-ALCAMID® IN BREAST: CASE REPORT
NEUMONITIS AGUDA SECUNDARIA A LA INYECCIÓN SUBCUTÁNEA DE GEL DE POLIALQUILAMIDA (BIO-ALCAMID) EN MAMAS: REPORTE DE CASO
Renzo Villanueva-Villegas
 1,2,
Anita Yesseña Trigoso Gutiérrez
1,2,
Anita Yesseña Trigoso Gutiérrez
 4,5,
Piero Castillo-Gutierrez
4,5,
Piero Castillo-Gutierrez
 4,5,
Juan A. Salas-López
4,5,
Juan A. Salas-López
 1,3
1,3
1Pulmonologist, Hospital Nacional Dos de Mayo. Lima, Peru.
2Instituto de Investigación de Ciencias Biomédicas (INICIB), Facultad de Medicina Humana. Universidad Ricardo Palma (URP). Lima, Peru.
3Teacher, Facultad de Medicina, Universidad Nacional Mayor de San Marcos (UNMSM). Lima, Peru.
4Estudiante de la Facultad de Medicina Humana, Universidad Nacional Mayor de San Marcos. Lima, Peru.
5Sociedad científica de San Fernando, UNMSM. Lima, Peru.
ABSTRACT
Filling materials are used to correct soft tissue deficits. Local and systemic complications are described when these substances are applied. The polyalkylamide gel (Bio-alcamid) used as filler material has been associated with complications at the facial level, while at the pulmonary level they have not been reported in the literature. We present the case of a patient who consulted due to sudden progressive dyspnea hours after the subcutaneous injection of Bio-alcamid in the breasts. After the clinical evaluation and the result of auxiliary tests, the diagnosis of acute pneumonitis secondary to the injection of this substance is concluded. Follow-up of the case was carried out with favorable evolution and resolution of symptoms after three months. We conclude that Bio-alcamid can generate complications at the pulmonary level, therefore, the intervention of public policies that regulate its use, commercialization and application by non-medical personnel is necessary.
Keywords: Acute pneumonitis, biopolymer, filler material, dyspnea Source: MeSH - NLM).
RESUMEN
Los materiales de relleno son utilizados para la corrección de déficit de tejido blando. Se describen complicaciones locales y sistémicas cuando se aplican estas sustancias. El gel de polialquilamida (Bio-alcamid) empleado como material de relleno ha sido asociado a complicaciones a nivel facial, mientras que a nivel pulmonar no han sido informadas en la literatura. Presentamos el caso de una paciente que consultó por presentar disnea súbita progresiva horas después de la inyección subcutánea de Bio-alcamid en mamas. Luego de la evaluación clínica y el resultado de exámenes auxiliares se concluye el diagnóstico de neumonitis aguda secundaria a la inyección de esta sustancia. Se realiza seguimiento del caso con evolución favorable y resolución de síntomas a los tres meses. Concluimos que el Bio-alcamid puede generar complicaciones a nivel pulmonar, por tanto, es necesario la intervención de políticas públicas que regulen su uso, comercialización y la aplicación por personal no médico.
Palabras Clave: Neumonitis aguda, biopolímero, material de relleno, disnea (Fuente: DeCS BIREME).
INTRODUCTION
Filling materials, such as biopolymers, have been used since the beginning of the 19th century to correct body defects for aesthetic or medical purposes (1). Bio-alcamid® is a biopolymer used as a non-resorbable and permanent filler material (1,2). It is mainly used at the facial level, so the complications reported on its use are mostly evident at this level (2). Until now, lung involvement secondary to the use of biopolymers as body shapers has been described in patients who received injection of dimethylsiloxane (silicone) (2), but not for those who used Bio-alcamid®. Based on the findings of clinical reports on silicone, biopolymers could generate four histological patterns that show lung damage, including acute pneumonitis (4). The pathophysiology is unclear, but it is postulated that this type of substances allogeneic to the organism can trigger cell-mediated immunity and activation of the humoral system with circulating antibodies (5). We report the case of a patient with acute pneumonitis after receiving a subcutaneous injection of Bio-Alcamid®.
CASE REPORT
36-year-old woman, native of Ancash, from Lima - Peru (Los Olivos) where she has lived since she was 5 years old, with a higher technical degree and currently dedicated to the trade of various products. He did not refer any significant pathological or family history, he denied alcohol, tobacco and drug use; He referred to doing sports daily in the gym and participating in marathon competitions in Lima, as well as in the interior of the country.
The patient went to the emergency room due to sudden progressive dyspnea, which occurred 8 hours after the subcutaneous injection of Bio-alcamid® for aesthetic use (breast lift) applied by non-medical personnel. After the procedure, the patient woke up with a feeling of marked shortness of breath (mMRC: 3) that was constant and which increased with the passing of the hours (mMRC: 4) without coughing or a sensation of thermal rise. The patient interpreted said symptoms as "a normal event" due to the application of biopolymer, so he did not decide to go for a medical evaluation.
After 4 days of rest at home and seeing that said symptoms persisted and made it impossible for him to carry out his activities, he decided to go to the pulmonology clinic for evaluation. His vital functions on admission were: BP: 100/60 mmHg, HR: 87 x min, RF: 28 x min, SatO2: 91% (FiO2: 21%), afebrile. On physical examination, the skin was warm, hydrated, with capillary refill of less than two seconds, no pallor, no central or peripheral cyanosis; in the respiratory system a symmetrical thorax is evident, no presence of retractions, preserved amplexation, increased vocal vibrations at the bases of both hemithoraxes, on auscultation vesicular murmur passes in both lung fields associated with scarce crackles in bilateral bases with a predominance of the anterior face of chest; the rest of the physical examination without alterations. Frontal and profile chest X-rays were requested, showing a basal bilateral heterogeneous radio parity with a predominance of anterior segments (Figure 1). The analysis of arterial gases at FiO2: 21% highlights an increase in the alveolar-arterial gradient (Gradient A-a: 28 mmHg), the rest of the exam was normal (pH: 7.44, PaCO2: 32.7 mmHg, PaO2: 81 mmHg, HCO3: 22.5 mmol/L, SatO2: 97%). The blood count was within normal parameters (Hb: 12.7 g/dL, Plaq: 204,000 cells/mm3, Leu: 9,700 cells/mm3, Eosi: 3%, Seg: 62%), but elevated PCR (48 mg /L). With these results, interstitial pneumonitis is presumed, so it is decided to hospitalize the patient to complement studies.

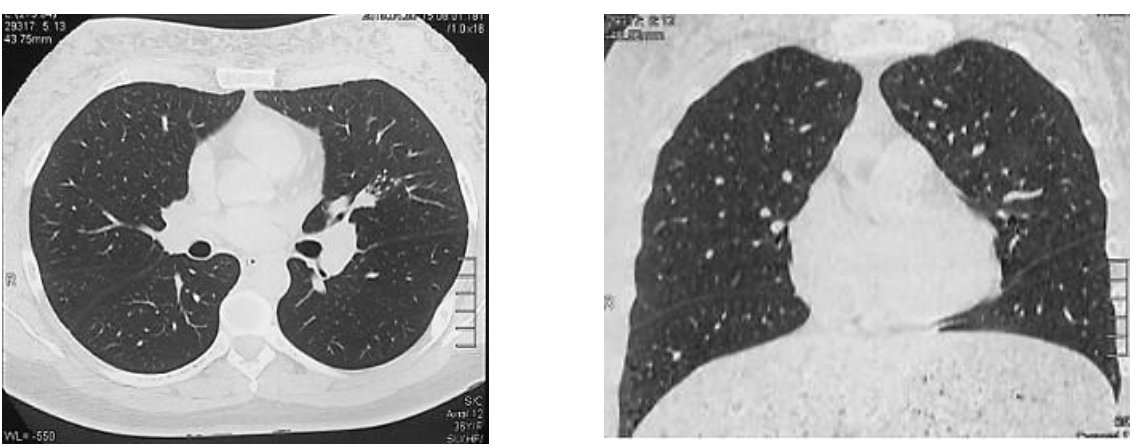
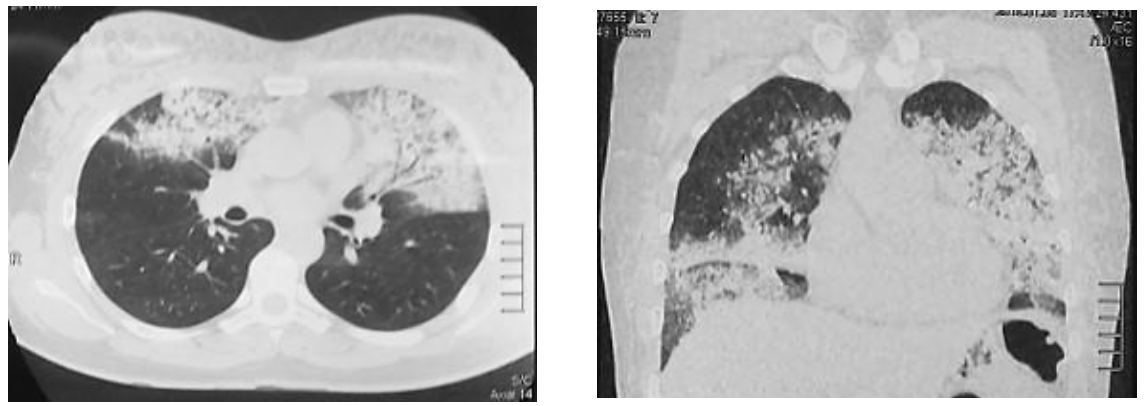
During hospitalization, the patient reported presenting cough with hemoptoic expectoration (dark brown), in addition to constant dyspnea (mMRC: 3) associated with oxygen saturation at FiO2 21% at rest between 91 and 93%, and when performing activities (talking fast or walking slowly on a flat surface) saturated 87%. A breast ultrasound was requested where anechoic collections were observed in both breasts, predominantly the left breast. Chest tomography shows consolidation lesions predominantly in the middle, lingula, and basal lobes, mainly in the anterior segments (Figure 2). A bronchoscopy was performed, where traces of bleeding were evidenced in the right lung in the upper lobe, segment 5 in the middle lobe with characteristic paleness of the mucosa, and the lower lobe without major alteration; on the contralateral side (left lung) traces of bleeding were evidenced in the upper lobe, followed by an incursion into the lingula where a sample of bronchial lavage, bronchial aspirate and transbronchial biopsy was taken; the rest of the procedure without alterations.
The transbronchial biopsy sample was reported as a lung fragment with a discrete interstitial mononuclear infiltrate and the presence of acellular eosinophilic material with peripheral histiocytic reaction. (Figure 3).

Based on the medical history and the results of the examinations, she was diagnosed as acute pneumonitis secondary to subcutaneous injection of Bio-alcamid® in the breasts. The patient presented a stationary evolution without complications, so hospital discharge was decided on the ninth day of hospitalization and follow-up by an outpatient clinic. At the first month of follow-up, he reported clinical improvement in dyspnea (mMRC: 2), and at the second month he resumed activities of daily living without difficulties (mMRC: 1). At the third month, there were no significant symptoms and the chest tomographic control showed complete resolution of the consolidations (Figure 4).
DISCUSSION
Polyalkylamide (Bio-alcamid®) is a synthetic and gelatinous polymer used as a non-resorbable and permanent filling material (1). Theoretically, this material has the property of inducing the host tissue to form a fibrous capsule around the deposit, thus preventing its infiltration (6). Among the main effects caused by this biopolymer, granulomatous reactions, nodular reactions with abscesses and superinfection stand out, which have been described mainly at the facial level, where it has its greatest applications(2,7). Pulmonary compromise has not been reported from the injection of this material.
Damage to the lungs secondary to the injection of biopolymers has rarely been described in the literature and its pathophysiology is poorly understood, but hypotheses have been raised based on dimethylxylosane or silicone, which is the biopolymer in most reported cases. The first is the increase in pressure and local tissue damage that favors the entry of the injected substance into the bloodstream and its embolization to the lung; while the second suggests that after the injection of the material, it is distributed in the alveolar space where inflammatory cells are recruited and circulating antibodies are activated that induce lung injury (4,8).
Lung involvement can manifest acutely, after hours or days from the injection; the symptoms are usually: dyspnea, tachycardia, tachypnea, fever, chest pain and hemoptysis, some patients can even reach acute respiratory failure (8,9). On the other hand, the latent form of lung involvement is after months or more than a year from the injection, affected patients presented edema and swelling at the site of application prior to respiratory symptoms that were described as mild and associated with hypoxemia (10). In this case, the patient presented sudden onset dyspnea, with no medical history that could explain the condition, and no complications were found at the injection site, which is consistent with the literature as an acute pulmonary complication.
For the diagnosis, especially when dealing with a picture of acute pulmonary involvement, it is important to suspect the cause and effect relationship between the history of injection of the biopolymer and the onset of the clinical picture. In addition, the presence of immunodeficiencies, infections, drugs or drugs as causes must be ruled out (8,9), which were negative in our patient.
Images show bilateral interstitial infiltrates with areas of non-uniform or “patchy” consolidation on radiography and computed tomography, where diffuse ground-glass images can also be seen (8). When it is not possible to take a sample for histological confirmation, bronchioloalveolar lavage (BAL) can be diagnostic since it typically shows increased cellularity at the expense of neutrophils, eosinophils and, to a greater extent, alveolar macrophages than inclusions are observed by electron microscopy. cytoplasmic cells containing foreign material (10). Histological patterns of lung damage that have been reported are the presence of intravascular silicone emboli, congestion and hemorrhage, diffuse alveolar damage, and acute pneumonitis (3); the latter was the pattern that presented the case in question.
Treatment is usually symptomatic, with rest, high-flow oxygen therapy if necessary, and in severe cases with ventilatory support (9). The use of corticosteroids does not have clear evidence of improving the evolution of this pathology, although some authors describe benefits of their use, especially when there is associated alveolar hemorrhage (11,12). In our case, we only intervened with symptomatic treatment and the patient maintained a stationary evolution during her hospitalization without actually requiring oxygen support; therefore, it was decided not to add another treatment.
CONCLUSION
Bio-alcamid® as a breast filler material can also generate complications at the pulmonary level, therefore, the intervention of public policies that regulate its use, commercialization and application by non-medical personnel is necessary.
Authorship contributions: RVV, AYTG, PCG and JASL have participated in the conception of the article, its writing and approval of the final version. In addition, RVV and JASL participated in the data collection. All authors take responsibility for the content of the article and agree to adequately answer any questions that may be necessary to ensure the accuracy of the data and integrity of any part of their research.
Funding sources: Self-financed.
Conflicts of interest: Renzo Villanueva-Villegas is supported by the Fogarty Research Training Scholarship in Noncommunicable Chronic Respiratory Diseases in Peru (PulmPeru), funded by D43 (D43TW011502). The rest of the authors declare that they have no conflict of interest.
Received: May 28, 2022
Approved: Ejemplo: January 21, 2023
Correspondence: Renzo Villanueva-Villegas.
Address: Jr. Jorge Chávez 1636 Dpto 703B Breña, Lima - Perú.
Telephone number: 956767552
E-mail: renzo.villanueva@urp.edu.pe
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
REFERENCES