CLINICAL CASE
REVISTA DE LA FACULTAD DE MEDICINA HUMANA 2023 - Universidad Ricardo Palma10.25176/RFMH.v23i4.5912
INTESTINAL OBSTRUCTION DUE TO STRONGYLOIDES STERCOLARIS: ABOUT A CASE.
OBSTRUCCIÓN INTESTINAL POR STRONGYLOIDES STERCOLARIS: A PROPÓSITO DE UN CASO
Karina Tarazona Ramírez
 1,b
1,b
Rolando Tapia Barcellandi
 1,a
1,a
Jesús De La Jara
 1,2,a
1,2,a
Enrique Vilchez Polo
 1,a
1,a
Orlando Aliaga Aliaga
 1,a
1,a
Pedro Sotelo Jiménez
 1,3,a
1,3,a
1 Hospital Nacional Edgardo Rebagliati Martins, Lima, Perú.
2 Facultad de Medicina, Universidad Nacional Mayor de San Marcos, Lima, Perú.
3 Facultad de Medicina, Universidad Ricardo Palma, Lima, Perú.
a Médico asistente.
b Médico residente.
ABSTRACT
Strongyloidiasis commonly causes gastrointestinal problems. We present the case of a male, a 30-year-old cadet in the Peruvian Navy from Lima, who developed a hyperinfection syndrome due to Strongyloides stercoralis, having a presumptive diagnosis of polymyositis for which he received a short cycle of corticosteroids. He was not a carrier of the HTLV 1/2 virus. Upon admission, he presented with hyporexia, generalized weakness, cachexia, intermittent self-limited diarrhea, oral intolerance, and mild abdominal distension. The patient reached the dissemination stage, resulting in severe intestinal damage. The low excretion of larvae in the feces made the diagnosis difficult. Treatment was provided with parenteral ivermectin at a dose of 1.2ml subcutaneously every 48 hours for three doses, with a good clinical response and subsequently good oral tolerance. The importance of presenting the case is to comment on the diagnostic and therapeutic approach to this endemic geohelminthiasis of Peru.
Keywords: Strongyloides stercoralis; ivermectin, cachexia, hyperinfection syndrome (Source: MeSH NLM).
RESUMEN
La estrongiloidiasis comúnmente produce problemas gastrointestinales. Presentamos el caso de un varón, cadete en la Marina de Guerra del Perú, de 30 años, procedente de Lima; que desarrolló síndrome de hiperinfección por Strongyloides stercoralis, teniendo como antecedente el diagnóstico presuntivo de polimiositis, por lo cual recibió un ciclo corto de corticoesteroides. No portador del virus HTLV 1/2. Presentó al ingreso hiporexia, debilidad generalizada, caquexia, diarrea intermitente autolimitada, intolerancia oral y leve distensión abdominal. El paciente llegó a la etapa de diseminación, lo que resultó en un daño severo a nivel intestinal. La baja excreción de larvas en las heces dificultó el diagnóstico. Se brindó tratamiento con ivermectina parenteral a dosis de 1.2ml vía subcutánea cada 48 horas por tres dosis, con buena respuesta clínica y posteriormente con buena tolerancia oral. La importancia de presentar el caso es comentar sobre el abordaje diagnóstico y terapéutico de esta geohelmintiasis endémica del Perú.
Palabras clave: Strongyloides stercoralis; ivermectina,caquéxia, síndrome de hiperinfección (Fuente: DeCS BIREME).
Strongyloidiasis is a parasitic disease caused by Strongyloides stercoralis, an intestinal nematode affecting between 30 and 100 million people worldwide (1). These worms typically nest in the small intestine, particularly in the duodenum, and in immunocompetent individuals, it tends to be asymptomatic or cause chronic intestinal form. In scenarios where the immune system is compromised, its clinical presentation can be very aggressive, leading to hyperinfection syndrome or dissemination of the nematode. It is endemic in Africa, Asia, Southeast Asia, and Central and South America, characterized by being highly prevalent in tropical and subtropical regions (1). According to a recent systematic review conducted in Peru, the prevalence in the general population is 7.34%, being more frequent in some regions such as Loreto, Ucayali, or Madre de Dios (2).
The disease has different forms of presentation. In its acute form, it primarily affects the first portions of the small intestine, causing manifestations such as alterations in defecation frequency, watery, mucoid, and even steatorrhea stools, constipation, epigastralgia, abdominal distension, and irritable bowel syndrome (3).
Human infection by Strongyloides stercoralis begins when the filariform larva, in its infective form, penetrates intact skin, which commonly occurs when people come into contact with contaminated soil while barefoot. Although most human infections result in a chronic asymptomatic condition of the gastrointestinal tract, the ability of Strongyloides stercoralis to complete its life cycle within the human host allows the parasitic load to significantly increase through a process of autoinfection. This mechanism underscores the critical role of the immune system in responding to the infection. In immunocompetent individuals, this interaction usually manifests as chronic intestinal infections. However, in those with immunosuppression, the autoinfection process favors the appearance of disseminated forms of the disease and hyperinfection syndrome. This phenomenon leads to the persistence and spread of the pathogen, exacerbated by a compromised cellular immunity response.
The aim of this article is to document a case of intestinal obstruction caused by a Strongyloides stercoralis infection, highlighting as the only risk factor a brief cycle of high-dose corticosteroids, administered over 8 days, that triggered a hyperinfection syndrome. Despite the patient not having visited jungle areas in approximately three years, this condition underscores the importance of considering clinical history and recent use of corticosteroids in the diagnosis and management of severe cases of hyperinfection by Strongyloides stercoralis. Moreover, it was necessary to use ivermectin via parenteral (subcutaneous) route to achieve patient recovery. This report emphasizes the relevance of disseminating clinical cases of rare pathologies, as it provides a valuable precedent for therapeutic management in the face of severe clinical manifestations of hyperinfection by Strongyloides stercoralis.
A 29-year-old male, originally from Iquitos, Loreto, and currently residing in Lima, where he has lived for the past 15 years, was admitted through the emergency department of the National Hospital Edgardo Rebagliati Martins (HNERM) in Lima, Peru, on April 17, 2023. The patient reported a trip to Iquitos three years ago, as well as serving as a cadet in the Peruvian Navy seven years ago, where he stayed for eight months between the Colombia and Peru border, in El Estrecho and Güepí, during 2015.
The patient reported a recent diagnosis of probable polymyositis, three months prior to admission. This diagnosis led to the use of prednisone 50 mg orally (po) every eight hours for three days, followed by prednisone 20 mg po every eight hours for five days, which was discontinued two days before admission on his own initiative.
Upon admission, a significant weight loss of 30 kg in the last five months (current weight: 28 kg) was noted, in addition to hyporexia, generalized weakness, and paresthesias in the lower limbs that limited walking. Furthermore, three months before admission, he experienced semi-liquid and intermittent diarrhea that resolved on its own after two months. Abdominal pain started one month prior, and 15 days before admission, he presented with persistent vomiting, mild abdominal distension, and intolerance to liquids and solids; he was admitted to the emergency department on May 2, 2023. The physical examination highlighted the presence of cachexia, diffuse abdominal pain upon palpation, and decreased muscle strength in the left half of the body.
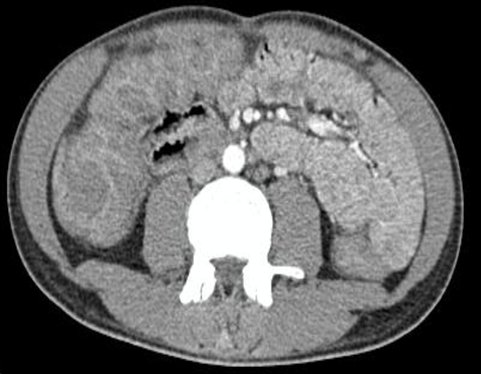
Complementary studies ruled out the diagnosis of polymyositis due to the absence of elevated CPK levels and an electromyography of the lower limbs without significant alterations. Additionally, a tomography showed mild hepatomegaly with diffuse edema of the intestinal loops with an inflammatory appearance in a water halo pattern (Figure 1).
Among the most significant findings from the laboratory tests, severe hypoalbuminemia with an albumin level of 1.7 g/dL and profound hyponatremia with sodium at 125 mmol/L stood out. Proteinuria in the non-nephrotic range was also observed, with a 24-hour urinary protein excretion of 1.13 g. The rest of the studies were within normal parameters (Table 1).
|
Laboratory Test |
Result |
Units |
|---|---|---|
|
Albumin |
1,7 |
g/dL |
|
Sodium (Na) |
125 |
mmol/L |
|
Potassium (K) |
4,19 |
mmol/L |
|
Creatinine (Cr) |
0,48 |
mg/dL |
|
Lactate Dehydrogenase (DHL) |
224 |
U/L |
|
B2 Microglobulin |
1,67 |
mg/L |
|
24-hour Urinary Protein |
1,13 |
gr |
|
Vitamin B12 |
>1000 |
pg/mL |
|
Homocysteine |
11,3 |
µmol/L |
|
Total IgE |
>2000 |
UI/mL |
|
IgG |
2942 |
mg/dL |
|
Tests for Porphyria |
Negativas |
- |
|
ANCA |
Negativo |
- |
|
ANA |
Negativo |
- |
|
Complement C3 |
En rangos normales |
- |
|
Complement C4 |
En rangos normales |
- |
|
Markers for infection by syphilis, Epstein-Barr virus, HIV, Hepatitis B and C, CMV, herpes simplex virus 1 and 2, HTLV 1/2 |
Negativos |
- |
|
Inflammatory Reaction in Stool |
Negativo |
- |
|
Thevenon in Stool |
Negativo |
- |
|
Bacilloscopy in urine, sputum, gastric aspirate, and stool |
Negativa |
- |
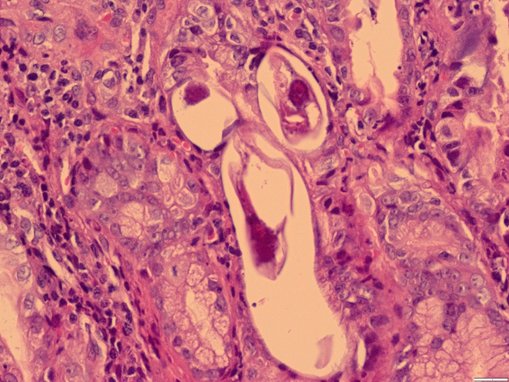
Due to persistent oral intolerance, an upper gastrointestinal endoscopy was performed, which described mild erythematous gastropathy and chronic duodenitis to be ruled out for inflammatory etiology, lymphoma, and Whipple's disease. The duodenal biopsy described alteration of the glandular structures by severe acute inflammatory infiltrate with the presence of polymorphonuclear leukocytes, eosinophils, and multiple parasitic structures whose morphology suggests Strongyloides sp. (Figures 2 and 3). The diagnosis was subsequently supported by parasitological examinations in stool, where the presence of Strongyloides stercoralis larvae was confirmed. Due to these results, in addition to clinical signs, the diagnosis of intestinal obstruction secondary to Strongyloides stercoralis hyperinfection syndrome was concluded.
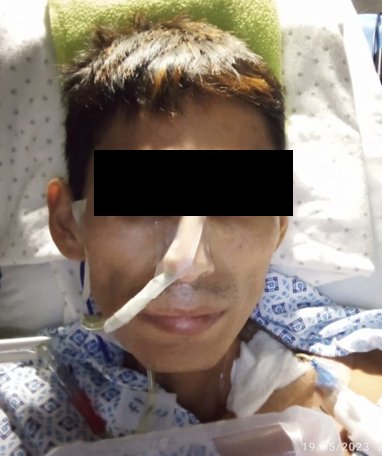
During hospitalization, the patient showed unsuccessful attempts to tolerate oral intake, severe hypoalbuminemia, and cachexia (Figure 4), for which parenteral nutrition was initiated. Given these findings, treatment with ivermectin via parenteral (subcutaneous) route was decided. As there is no presentation for use in humans, it was agreed in a clinical meeting with the service doctors, with prior consent from the patient and family, to use veterinary ivermectin at a dose of 200 µg/kg (1.2 ml) subcutaneously (s.c) every 48 hours for three doses. Additionally, evacuant enemas and placement of a nasogastric tube by gravity were applied due to the intestinal obstruction. During the treatment, the patient did not present any adverse events.
The patient showed good clinical and microbiological response. After 7 days of starting the antiparasitic treatment, the patient began an oral diet with good tolerance, remitting episodes of nausea and vomiting, as well as abdominal pain. The patient was discharged after having been hospitalized for 57 days, leaving with a weight at the last control of 67 kg (weight delta of +32 kg). Finally, he returned to his regular activities without difficulty.
DISCUSSION
This case presents a patient with epidemiological risk factors and the use of a short cycle of high-dose corticosteroids leading to hyperinfection by Strongyloides sp., which, following an early diagnostic and alternative therapeutic approach with subcutaneous ivermectin, showed clinical and microbiological improvement in the intestinal obstruction scenario. (4) For the prescription of non-human use subcutaneous ivermectin, we relied on studies conducted as detailed by Soto-Febres et al.; Barret Jessica et al.; Zeitler Kristen et al., and Hennessey Diana et al. (3, 10, 5, 6), where doses of non-human use subcutaneous ivermectin were used without adverse reactions and/or serious complications.
Strongyloidiasis is a curable disease where early diagnosis and appropriate therapy can reduce mortality. Anyone with a history of travel or residence in an endemic area of the disease, even decades before presentation, should be screened for strongyloidiasis if they present compatible symptoms or if they are asymptomatic but are candidates to receive corticosteroids or other immunosuppressive therapy in the near future. If the patient is immunocompromised, the nematode's autoinfection cycles intensify, and the number of parasites increases considerably, which can lead to hyperinfection syndrome. (7) Hyperinfection by Strongyloides stercoralis often occurs in patients with some degree of immunosuppression. It is frequently associated with co-infection by HTLV-1/2. Additionally, the literature describes predispositions in patients with multiple myeloma, allogeneic stem cell transplantation, Crohn's disease, myasthenia gravis, ANCA+ crescentic glomerulonephritis, malnutrition, lymphoma, renal transplants, chronic alcoholism, and the use of steroids, rituximab, and interferon as risk factors. (7) In the case presented, infections such as HTLV-1/2 and HIV, as well as other risk factors, were ruled out. The only predisposing factor was the short cycle of high-dose corticosteroids the patient received for a presumptive diagnosis of polymyositis. It is noteworthy that the short duration of prednisone administration was a risk factor for the development of hyperinfestation, likely due to the high doses. However, there have been reports that even very short cycles of corticosteroids have precipitated this condition. (8)
The hyperinfection syndrome in the patient described manifested clinically with gastrointestinal involvement, including protein-losing enteropathy and diarrhea with abdominal pain, explaining the malnutrition and associated hypoalbuminemia. (9)
In the case we present, the patient never had eosinophilia; instead, he exhibited eosinopenia. In other cases, co-infection with HTLV-1/2 or HIV is common, but our patient did not have these co-infections. The immunosuppression was due to corticosteroids used erroneously without clinical or histopathological basis. If the patient is immunocompromised, these autoinfection cycles intensify, and the number of parasites increases considerably, which can lead to hyperinfection. Moreover, this hyperinfection itself can alter the host's immune response, inhibiting the production of eosinophils because the accelerated life cycle of the parasite and its increased dissemination interfere with the immune system's ability to mount the characteristic eosinophilic response of parasitic infections. Thus, in the study by Chen YA et al., eosinophilia is mentioned as an indicator of infection in risk populations but is not sensitive enough to be used as a sole monitoring marker in uncomplicated chronic infection due to its intermittent presence. (10) It is reported that eosinophilia might even be absent in the syndrome of hyperinfection by Strongyloides. (11)
Regarding other cases and their treatment in the study by Hennessey D. et al., the case of a 31-year-old female patient with a history of renal transplantation, who presented with epigastric pain associated with vomiting and was diagnosed with partial intestinal obstruction. After a multidisciplinary medical board, she was administered veterinary use subcutaneous ivermectin, in doses of 1.2 ml (12,000 µg or 12 mg) every 48 hours, for a total of three doses, with which the patient had an adequate evolution. (12) Another similar case by Soto-Febres F. et al., which occurred in Peru, where a patient from the central jungle (Pichanaqui, Junín) developed abdominal distension and weight loss, later adding symptoms of high intestinal obstruction. After a range of studies, a foreign body in the stomach and duodenum was found, with the presence of larvae and adult forms of S. stercoralis, which was associated with a state of immunosuppression by HTLV-1/2 and was treated with oral ivermectin. (3)
It is important to highlight that the treatment of hyperinfection by Strongyloides sp. is based on the administration of anthelmintics, mainly ivermectin, other options being thiabendazole or albendazole. (13) In Peru, Colombia, and worldwide, however, there is no parenteral ivermectin presentation for use in humans, which constitutes a problem in those patients with compromised enteral absorption due to, for example, an intestinal obstruction, hence the choice for S.C. treatment in cases of intestinal obstruction with oral intolerance as happened with our patient, making it one of the few cases reported treated and reported with S.C. ivermectin in Peru with good microbiological and clinical response; similar to a previous report where transmission of Strongyloides stercoralis from a donor to two renal transplant recipients caused gastrointestinal, respiratory, and dermatological symptoms. The diagnosis was confirmed by larvae in respiratory and duodenal samples. Treatment with albendazole and oral ivermectin was not successful for one; while the other survived with subcutaneous ivermectin, but with intestinal failure. (14) Additionally, as mentioned, the patient had a favorable evolution of the disease in his outpatient control.
It is important to detect Strongyloides sp. infection in patients about to begin immunosuppressive therapy and who have previously visited or lived in endemic regions (Southeast Asia, South America, Africa). The patient presented certain risk factors such as epidemiological origin, hypoalbuminemia, and the use of corticosteroids that led to hyperinfection by Strongyloides sp.
One of the limitations presented by the case is that the patient arrived with a diagnosis of probable polymyositis that was not verified upon admission because we did not have access to the medical report of that diagnosis and what specific treatment was received. However, at HNERM, complementary tests such as electromyography and the dosage of the muscle enzyme creatine kinase (CPK) were performed, which are sufficient data to rule out such a diagnosis. Another limitation was that initially, the patient had proteinuria in the 24-hour urine sample, so a renal biopsy was proposed to assume that the proteinuria was due to the hyperinfection by Strongyloides sp. It was not performed, however, a urine analysis control after the received treatment was carried out, where proteins were searched in the 24-hour urine sample whose result was within the normal range.
This case is important because it shows an experience of subcutaneous administration of ivermectin, which is a non-conventional therapy in Peru that was successful and without immediate systemic adverse effects in our patient, as not many experiences with the use of subcutaneous ivermectin have been reported in Peru compared to other clinical cases reported elsewhere in the world. With this, we recommend for possible replications of use as a therapeutic alternative in similar patients with hyperinfection with Strongyloides sp. who do not tolerate the oral route and who suffer from intestinal obstruction. In addition, we also recommend the performance of subsequent studies on the pharmacokinetics or pharmacodynamics of ivermectin for subcutaneous use in humans that help determine the behavior of ivermectin, to establish the frequency of its administration, the maximum tolerated dose and the safe dose in humans, among other relevant data. The benefit, apart from the unconventional therapy, is that the hospital, being a national reference, has an artificial nutritional support unit (USNA), which provided the patient with part of the multidisciplinary management with total parenteral nutrition (TPN) and enteral nutrition throughout his stay and did not require surgery.
In conclusion, strongyloidiasis is a neglected disease in Latin America; that can present with nonspecific gastrointestinal manifestations, being duodenal obstruction an infrequent complication that can be an index of mortality, if this disease is not diagnosed and treated in time; hence reporting the importance of strategies from the diagnostic approach, such as the tests that can be used for its confirmation: parasitological examination, duodenal mucosa biopsy plus the clinical manifestations of high intestinal obstruction would confirm the diagnosis and early treatment with ivermectin would help avoid its complications.
It is also worth highlighting the importance of the epidemiological history and the risk factors associated with this entity will guide us towards better management of this disease and thus reduce the associated mortality rate (15).
Authorship contributions:
All authors have participated in conceptualization, methodological design, data analysis, writing and revision of the manuscript.
Financing:
Self-nanced
Declaration of conflict of interest:
The authors declare that they have no conflicts of interest.
Recevied:
September 12, 2023
Approved:
December 22, 2023
Correspondence author:
Karina Patricia Tarazona Ramírez
Address:
Av. Jose Galvez 1212 – Santa Beatriz – Cercado de Lima.
Phone:
(+51) 956 431 874
E-mail:
karina.tarazona.ramirez@gmail.com
Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
BIBLIOGRAPHIC REFERENCES