INTRODUCTION
Iron deficiency and the consequent anemia represent a major public health problem, impacting all stages of life, especially in vulnerable populations, with significant repercussions in developing countries. According to the World Health Organization (WHO), the global prevalence of anemia is 40% in children aged 6 to 59 months and 37% in pregnant women. In Peru, the prevalence of anemia in children aged 6 to 35 months reaches 40.1%
1
. These figures highlight the magnitude of the problem and the need for research aimed at understanding its causes, consequences, and possible interventions.
Iron is an essential micronutrient for the body, as it is involved in various biological processes, including the transport of oxygen through hemoglobin, DNA synthesis and repair, mitochondrial function, and cellular energy production
2
. Its deficiency can lead to multiple complications, including dyspnea, headaches, fatigue, restless leg syndrome, and decreased physical and mental performance
3
. These manifestations not only affect the individual's quality of life but also generate social and economic repercussions
4
.
Given the need to deepen the study of the consequences of iron deficiency, it is essential to have experimental models that facilitate the research of the factors associated with this condition. Although there are studies describing the experimental induction of iron deficiency in animals, many of them do not accurately detail the dietary formula used or rely on complex procedures to achieve this goal
5, 6
. These limitations highlight the need to develop accessible and standardized protocols for inducing anemia in preclinical models.
The present study aimed to develop an experimental model of anemia in Balb/c mice through an iron-deficient diet to provide suitable preclinical models for the study of various variables associated with anemia.
METHODS
An experimental and longitudinal study was conducted at the animal facility of the Faculty of Medicine of the Universidad Nacional Mayor de San Marcos (UNMSM). The study included 16 male Balb/c mice, approximately four weeks old, acquired from the Instituto Nacional de Salud in optimal sanitary conditions and with appropriate sizes and weights for their age. The animals were acclimatized for three days with a standard diet for mice before beginning the experimental phase. They were then randomly divided into two groups of eight animals each: group A, which continued with the standard diet, and group B, which received an iron-deficient diet.
To evaluate the development of experimental anemia, two main indicators were used: hemoglobin level and body weight in both groups of animals. These parameters were recorded at the start of the experiment and at 30 days, following methodologies described in previous studies
8
. Hemoglobin levels were determined using an EKF Diagnostic® hemoglobinometer, based on optical absorption photometry by the methemoglobin azide method. Blood samples were collected by making an incision at the distal end of the mouse's tail with a No. 10 scalpel blade, discarding the first drop and using the second for analysis
10, 11
.
Experimental anemia was induced using an iron-deficient diet, whose composition was based on previous studies
7, 8
. The formula consisted of 100 g of milk mix, to which 4 g of cornflour and 2 g of powdered wheat bran were added. This diet had an iron concentration of 2.83 mg/Kg (Table 1).
Table 1. Organoleptic and physicochemical characteristics of the milk formula used
| Parameter |
Result |
| Taste |
Milky |
| Color |
Creamy white |
| Consistency |
Powder |
| Protein |
22.00% |
| Total fats |
24.00% |
| Ash |
Max. 7.00% |
| Titratable acidity expressed as % lactic acid |
0.10 - 0.15 |
Source: Technical sheet of the milk blend from Danilac® E.I.R.L.
The standard diet for mice was obtained from the Universidad Nacional Agraria La Molina and consisted of cornflour, soy cake (48% protein), extruded whole soy flour, wheat by-products, soybean oil, calcium carbonate, dicalcium phosphate, choline chloride at 60%, sodium chloride, synthetic amino acids, vitamin-mineral premix, antioxidants, and antifungals
9
, with an iron concentration of 205.59 mg/Kg. The analysis of both diets was conducted in a certified laboratory, with test reports No. DT-03581-01-2023 and No. DT-03581-03-2023, respectively.
Hemoglobin was measured in blood samples obtained from the caudal vein of the mice using the procedure described in Figure 1. An incision was made at the distal end of the tail with a No. 10 scalpel blade, discarding the first drop of blood and using the second drop collected in a microcuvette for analysis in the hemoglobinometer
12, 13
.
Figure 1. Procedure for hemoglobin measurement: A) The tail is held and asepsis is performed using a cotton swab soaked in 70° medicinal alcohol. B) An incision is made in the distal part of the tail using a No. 10 scalpel blade and a No. 03 scalpel handle. C) Pressure is applied to the tail to obtain the first drop, which is discarded. D) The second drop is obtained and placed in the hemoglobinometer cuvette. E) The cuvette is inserted into the equipment, and the reading is performed.
The evaluation of anemia at 30 days was conducted considering the time proposed in previous studies for anemia induction, particularly the works of Amaro-Terrazos et al. and Gonzales-Carazas et al., whose experimental groups included a group with iron deficiency (ID) and a group with iron deficiency and Erythroxylum coca extract (ID-EC), respectively
7
8
.
The animals' weight was recorded at the same time points using a Soehnle® digital scale.
The data were analyzed using GraphPad Prism software, version 10.4.1. Normality of the data was assessed, and based on the results, the Student's t-test for independent samples was applied. A significance level of 0.05 was considered. The study was conducted following ethical guidelines for research involving laboratory animals. Project approval was obtained with code N° A23012381-RR 006081-R-23/UNMSM and the ethical approval certificate from the Faculty of Medicine of UNMSM with number N° 0155-2023.
RESULTS
In the initial evaluation, no significant differences were found in either of the two indicators. However, there was a significant statistical difference at 30 days when comparing hemoglobin levels and weight between group A vs. group B, as shown in Table 2 after applying the t-test for independent samples.
Table 2. Comparison of hemoglobin levels and weight between Balb/c mice with standard diet (A) and iron-deficient diet (B).
| Indicator |
Group |
Baseline measurement (Mean ± SD) |
Measurement at 30 days (Mean ± SD) |
| Value |
p-value |
Value |
p-value |
Hemoglobin
(g/dL) |
A |
13.61 ± 1.06 |
0.442 |
15.65 ± 0.96 |
<0.001* |
| B |
14.20 ± 0.66 |
|
5.30 ± 1.67 |
|
Weight
(g) |
A |
11.61 ± 1.39 |
0.4504 |
38.13 ± 2.167 |
<0.003* |
| B |
12.32 ± 2.82 |
|
28.63 ± 6.19 |
|
* Statistically significant difference. t-values: 1.56 (baseline hemoglobin comparison), 15.21 (30-day hemoglobin comparison), 0.4504 (baseline weight comparison), 4.099 (30-day weight comparison). SD: standard deviation.
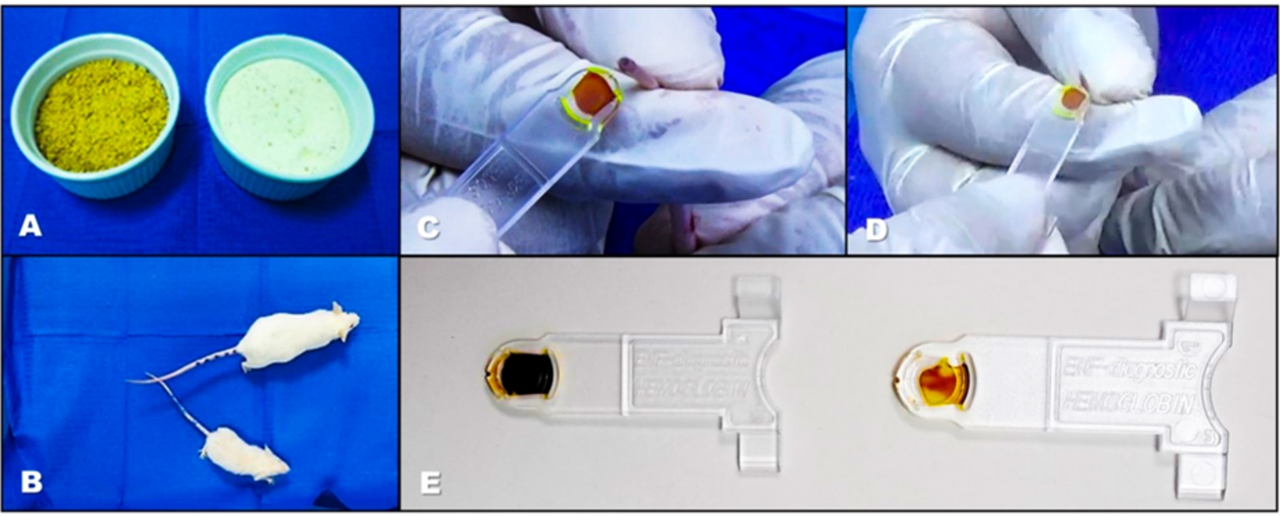
The conventional diet was ground with the aim of achieving the same consistency as the iron-deficient diet, which was presented in powder form, as shown in Figure 2. This figure also shows the visible weight differences between one animal from each group at 30 days, with a paler color observed in the cuvettes of the samples from the group subjected to the iron-deficient diet.
Figure 2. Macroscopic differences between an animal on a normal diet vs. an iron-deficient diet. A) Diets applied, the brown diet is the normal one and the white one is the iron-deficient diet. B) Visible differences in weight and size between a representative from each group. C-E) Clear differences in the coloration of the blood samples, with the palest ones corresponding to the animal on the iron-deficient diet.
Discusión
In the initial evaluation, no significant differences were found in the baseline levels of weight and hemoglobin between the experimental groups. However, after 30 days of administration of the standard diet and the iron-deficient diet, significant differences were observed in both parameters. Additionally, a marked contrast in the coloration of the blood obtained from the groups was evident, suggesting a direct effect of iron restriction on erythropoiesis. These results confirm that the preclinical model used is effective in replicating iron-deficiency anemia conditions, which will allow the study of its molecular and physiological consequences.
The administration of the iron-deficient diet significantly impacted hemoglobin levels, as evidenced by the 30-day measurement, where values were 15.65 g/dL in group A and 5.30 g/dL in group B. This suggests that the dietary formula used was effective in inducing anemia, which is attributed to its low iron content (2.83 mg/Kg). Previous studies in rodents have reported a significant reduction in hemoglobin following the administration of iron-deficient diets, in line with the findings of the present study
5
7
. This decrease is due to iron restriction in the diet, which alters erythropoiesis, a fundamental process in the production of erythrocytes
14
.
Regarding body weight, a significant difference was observed between the experimental groups after 30 days of intervention (38.13 g in group A vs. 28.63 g in group B). It has been reported that iron deficiency in early stages can affect growth
15
16
, ), which is consistent with our findings. This alteration could be related to reduced food intake, a phenomenon observed in previous studies where anemia decreases appetite and physical activity, affecting body weight. Furthermore, iron deficiency could dysregulate ghrelin concentration, a key hormone in the regulation of energy metabolism, which in turn impacts growth
17
18
.
A preclinical study that induced iron deficiency anemia and later reintroduced this micronutrient into the diet showed that animals with iron deficiency weighed less than the control group, and this difference persisted even after iron repletion
19
. These findings support the importance of iron as an essential nutrient for early growth and development. Additionally, anemia generates a hypoxic condition in hepatocytes, inhibiting the action of insulin-like growth factor 1 (IGF-1) due to the increase in its binding protein (IGFBP-1)
15
. Since IGF-1 is a hormone involved in bone growth at the epiphyseal plates of long bones, its inhibition can affect normal development
20
.
In the present study, these biological events were reflected in the size differences between the experimental groups.
Regarding the coloration of the blood, hemoglobin consists of a globin and a heme prosthetic group, which contains iron and porphyrin, giving it its characteristic red color
3
.In this study, iron deficiency caused a clear difference in the pigmentation of the blood obtained from the mice, with those fed the standard diet having darker coloration compared to those fed the iron-deficient diet.
The implications of this study are related to the high prevalence of iron-deficiency anemia worldwide and its impact on cognitive, physical, and metabolic development. These findings highlight the importance of continuing preclinical research to expand and deepen knowledge in this area, with the goal of developing effective strategies for the prevention and treatment of this condition
21
. Furthermore, it would be relevant to replicate this study in female animals to evaluate potential differences in response to iron deficiency.
Among the strengths of the study is the implementation of a simple and accessible method to induce anemia through an iron-deficient diet, made with commercially available inputs, facilitating its replication. Additionally, accessible and easily standardized instruments and equipment were used, ensuring the feasibility of the procedure.
However, among the study's limitations, it should be noted that the dairy blend used as the base for the iron-deficient diet is produced by a Peruvian company, which could hinder its replication in other countries. Furthermore, the inclusion of a larger number of animals and evaluations at different time points (15, 45, and 60 days) would have provided a more comprehensive view of the anemia induction process.
In conclusion, the implementation of an iron-deficient diet allowed the development of an experimental anemia model in four-week-old Balb/c mice at the 30-day evaluation. This model constitutes a simple and reproducible methodology to induce anemia in a preclinical setting, facilitating the study of various variables associated with this condition.
ACKNOWLEDGEMENTS:
To Dr. Miguel Hernán Sandoval Vegas for his support in the experimental aspect and to Mr. Reinaldo Madrid Prado, who was in charge of the care and maintenance of the experimental animals.