Introduction
Growing teratoma syndrome (GTS) is defined by the enlargement of tumors during or after chemotherapy for the treatment of non-seminomatous germ cell tumors. This phenomenon is accompanied by the normalization of previously elevated tumor markers and the presence of mature teratoma components on histological examination.
1
2
Although the exact etiology of GTS remains unclear, its clinical features are well established. Due to its resistance to both chemotherapy and radiotherapy, surgical intervention is the main curative approach.
3
This syndrome was first documented in 1982 by Logothetis; however, its relative rarity has contributed to a lack of medical awareness, potentially delaying diagnosis and treatment, thereby increasing the risk of complications.
4
The incidence of GTS in patients with non-seminomatous germ cell tumors ranges from 1.9% to 11.7%. Growing tumors are typically located in the pelvis, abdomen, or retroperitoneum, although they have been less frequently documented in lymph nodes and lungs. The objective of this case report is to describe the clinical, surgical, and histopathological features of a patient with GTS presenting as a retroperitoneal mass, highlighting the diagnostic and therapeutic challenges associated with this condition.
Case Report
The patient, a 25-year-old male from Moyobamba and resident of Lima, had a history of deep vein thrombosis in the right leg, treated with enoxaparin. He presented with swelling in the right testicle. In December 2022, he was diagnosed with a primary non-seminomatous germ cell tumor of the right testicle at clinical stage IIIB (T2 N3 M0 S2). He underwent a radical right orchiectomy, revealing a solid 7 cm tumor with a stony appearance and no scrotal infiltration during surgery. Pathological analysis indicated a mixed germ cell tumor composed of 30% yolk sac tumor, 50% embryonal carcinoma, and 20% mature teratoma, with vascular infiltration of the tunics. The margins of the spermatic cord were free of neoplasia.
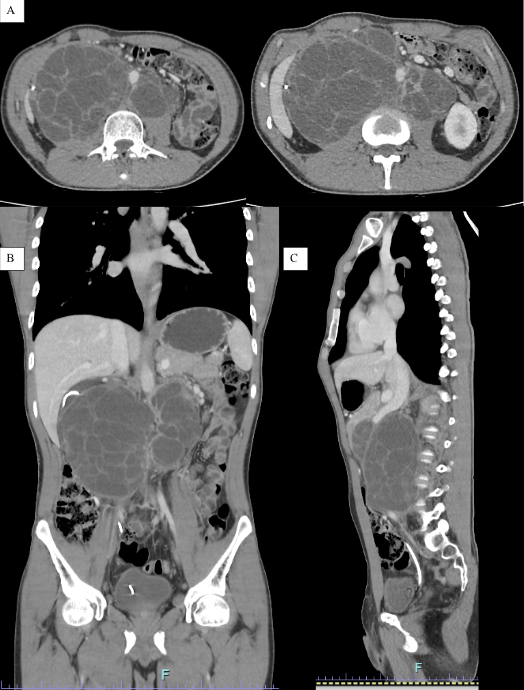
Subsequently, the patient received four cycles of BEP chemotherapy (bleomycin, etoposide, and cisplatin). In May 2023, apparent progression of retroperitoneal disease was detected. CT scans revealed the presence of three septated cystic lesions in the retroperitoneum: one in the right psoas muscle (15x11x11 cm), another in the left psoas muscle (11x7x5 cm), and a third in the mesentery (6.5x3.6x6 cm), compressing the inferior vena cava. Additionally, lymphadenopathy was observed in the right para-aortic region (17x13x27 mm) and left para-aortic region (25x10x19 mm), along with moderate to severe hydronephrosis in the right kidney and parenchymal thinning of 12 mm (Figure 1). This prompted treatment with TIP chemotherapy (paclitaxel, ifosfamide, and cisplatin) for three cycles.
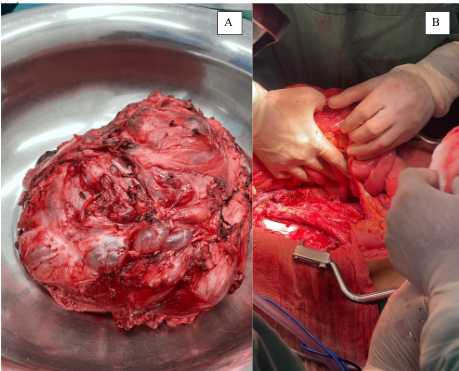
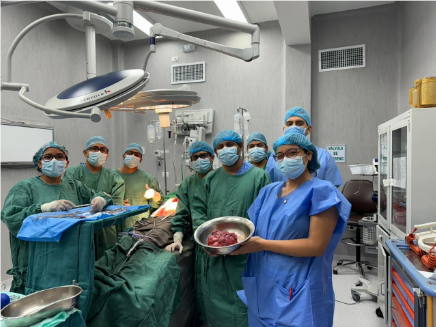
Following a medical board deliberation, exploratory laparotomy, retroperitoneal lymphadenectomy, and mass resection were decided upon (Figure 2). This high-complexity procedure extended beyond six hours, necessitating the patient’s subsequent transfer to the intensive care unit (ICU) for continuous evaluation. This outcome was achieved through the collaborative efforts of specialists in oncological surgery sub-specialties, including urologic oncology, thoracic surgery, and oncological abdominal surgery (Figure 3). The patient recovered well from surgery and, after a period of comprehensive care and observation, was discharged from the hospital.
The resected lymphadenopathy and retroperitoneal mass were sent for analysis, and pathological examination confirmed the presence of mature teratoma, clinically consistent with GTS based on laboratory findings.
Studies involving human participants were reviewed and approved by the Ethics Committee of the Oncology Department of the Hospital Santa Rosa. The patient provided written informed consent to participate in this study.
Discussion
The presented case highlights the complexity of GTS, a rare but potentially serious condition that can arise after treatment of non-seminomatous germ cell tumors. GTS is characterized by the progressive growth of tumors, particularly in retroperitoneal regions, even after chemotherapy. Previous studies have documented the resistant nature of GTS to conventional treatments, emphasizing the importance of alternative therapeutic approaches.
5
6
An increase in tumor mass or lesion number following chemotherapy is considered indicative of ineffective treatment, a common situation in clinical settings. A lack of understanding and consideration of this phenomenon often contributes to its presentation. GTS is characterized by progressive tumor growth despite the normalization of serum markers, and its diagnosis depends on the exclusive identification of mature teratoma components in histopathology.
7
Although rare, GTS has been reported in both testicular and ovarian tumors, suggesting a shared but poorly understood pathophysiology.
8
In this 25-year-old patient, despite initial surgery and multiple cycles of chemotherapy, disease progression was evident with the appearance of a retroperitoneal mass. This clinical course is consistent with findings previously reported in the literature, highlighting the tendency of GTS to persist or recur even after surgical resection and cytotoxic therapy.
6
The literature emphasizes that recurrence often occurs in surgically challenging areas, such as the pelvis or retroperitoneum, increasing management difficulties.
9
Early detection and appropriate management of GTS are crucial due to its resistance to conventional therapy and its potential to cause serious complications. In this situation, exploratory laparotomy and retroperitoneal lymphadenectomy were chosen to address the mass and lymphadenopathy. Pathological analysis confirmed the presence of mature teratoma components, corroborating the diagnosis of GTS, consistent with previous studies highlighting teratomatous tissue in GTS cases.
5
The literature indicates that in such cases, surgery is the treatment of choice, with complete resectability being essential to prevent further recurrences.
9
10
It is important to emphasize that GTS poses diagnostic and treatment challenges due to its rarity and lack of clinical recognition. The diversity in clinical presentations and the absence of specific markers often delay diagnosis, which can negatively influence the patient’s prognosis. Therefore, increasing awareness among healthcare professionals about GTS and considering it in the evaluation of patients with a history of non-seminomatous germ cell tumors who experience tumor growth after treatment is essential.
Diagnosing GTS presents a considerable challenge for oncologists due to the rarity of the condition. However, early diagnosis can prevent patients from undergoing costly tests, chemoradiotherapy, and major surgical procedures. The diagnosis is confirmed using the criteria established by Logothetis, which include normalization of serum markers, observed increases in tumor size or lesion number (or both) on serial imaging during or after chemotherapy, and the exclusive identification of mature teratoma components in surgical specimens from patients with known histories of non-seminomatous germ cell tumors.
4
10
Moreover, further research at the national and international levels is needed to better understand the underlying pathophysiology of GTS and develop more effective therapeutic approaches. This includes identifying predictive biomarkers for treatment response and exploring therapies specifically targeting the mechanisms responsible for persistent tumor growth in GTS.
Conclusion
This case highlights the importance of maintaining a high index of suspicion for GTS in patients with a history of non-seminomatous germ cell tumors and emphasizes the need for a multidisciplinary approach involving oncologists, surgeons, and pathologists to achieve optimal management of this condition. Comparing this case with similar studies at the national and international levels could provide valuable insights into trends, treatment approaches, and long-term outcomes of GTS across different populations and clinical settings.
Figure 1
Abdominal and pelvic computed tomography study showing septated cystic lesions in the retroperitoneum. A) Axial plane. B) Coronal plane. C) Sagittal plane.
Figure 2
Surgical procedure for retroperitoneal mass. A) Resected retroperitoneal mass. B) Outcome of the resection of the retroperitoneal mass, lymphadenopathy, and closure of the inferior vena cava.
Figure 3
Multidisciplinary team from the Oncology Department of "Hospital Santa Rosa," comprising specialists in Oncological Surgery and subspecialists in Oncological Urology and Oncological Abdominal Surger