INTRODUCTION
Coronaviruses use the homotrimeric spike glycoprotein of their envelope to bind to their cell receptors through membrane fusion, thus allowing their entry. Binding to the angiotensin II receptor (ACE2) is a critical initial step for SARS-CoV-2 to enter target cells, meaning that cells expressing ACE2 are susceptible to infection
1
. Endocrine alterations have been described in COVID-19, and pituitary and hypothalamus lesions are frequent. The dissemination route can be hematogenous, through leukocytes as a vehicle to the central nervous system crossing the blood-brain barrier, or neural, mediated by ACE2 receptors in the vascular endothelium
2
. Direct alterations of the virus by immune-mediated responses mainly at the pituitary gland generate lesions, low availability of vasopressin, secondary polyuria, and hypernatremia
3
.
Diabetes insipidus (DI) is a condition characterized by an imbalance of water in the body, leading to intense thirst and polyuria. Central diabetes insipidus (CDI) occurs when there is a deficiency in the secretion of vasopressin (antidiuretic hormone) from the pituitary gland. The hypothalamic-pituitary axis can be affected by various etiologies, including infections like COVID-19, which may hypothetically lead to CDI due to direct viral invasion or immune-mediated damage.
COVID-19 and hypernatremia: Hydroelectrolyte disorders are frequent in intensive care units. It has been found that hypernatremia has a prevalence of approximately 26 %
1
. However, reviews in patients with SARS-CoV-2 have a much higher presentation (50 %), associated with higher morbidity, days of mechanical ventilation, and length of ICU stay
2
. In a series of approximately 10,000 patients, most records correspond to hyponatremia. However, patients with hypernatremia, whatever their level, had higher mortality than eunatremic or hyponatremic patients
3
.
CASE REPORT
A 46-year-old woman with a history of arterial hypertension managed with angiotensin II receptor antagonists and hydrochlorothiazide presented with general symptoms and progressive dyspnea, testing positive for COVID-19 by PCR. She required orotracheal intubation and admission to the intensive care unit (ICU). Nine days after the onset of symptoms, she developed hypernatremia and was managed with hypotonic intravenous fluids and free water by orogastric tube. However, four weeks later, she developed refractory hypernatremia to the established management (sodium 190 mEq/L). Therefore, the patient also required continuous slow hemodialysis for its correction.
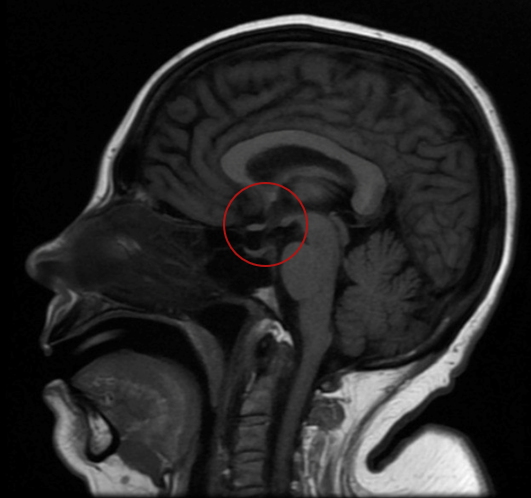
During her hospitalization, the patient's urine output was documented to be excessively high, supporting the diagnosis of polyuria. Laboratory tests documented hyposthenuria, high serum osmolarity, and low urinary osmolarity corrected with desmopressin administration, indicating central diabetes insipidus. Furthermore, during evolution, abrupt fluctuations in serum sodium levels were presented (higher than 16 mEq/L in 24 hours), associated with neurological deterioration without pathological findings in simple brain tomography. Magnetic resonance imaging (MRI) was requested (figure 1), documenting a neurohypophysis hypointensity concerning diabetes insipidus of central origin, associated with changes due to extrapontine osmotic demyelination.
The patient was on mechanical ventilation throughout her ICU stay and did not experience cardiac arrest. She was weaned off mechanical ventilation as her condition stabilized. After a prolonged ICU stay of five weeks, she was transferred to a general ward. Her sodium levels gradually stabilized with the continued administration of desmopressin. The patient was discharged home on desmopressin therapy.
DISCUSSION
We present the case of a female patient in her fifth decade of life who, during a COVID-19 infection, developed hypernatremia refractory to management with hypotonic solutions, even requiring dialytic therapy for its control. During its study, high serum osmolarity and low urinary osmolarity were documented, which responded to desmopressin management, indicating central origin diabetes, explained by 100% recovery of urinary osmolarity. However, fluctuations in serum sodium led to extrapontine osmotic demyelination, the consequences of which have limited the release of mechanical ventilation.
Hypernatremia in COVID-19 patients poses significant clinical challenges, particularly in those requiring intensive care unit (ICU) management. Our case highlights the complexity of managing hypernatremia in the context of COVID-19 infection and underscores the importance of considering various underlying mechanisms.
Renal mechanisms
Renal dysfunction associated with COVID-19 may contribute to hypernatremia through several mechanisms. The expression of angiotensin-converting enzyme 2 (ACE2) receptors in the proximal tubule facilitates SARS-CoV-2 entry into renal cells, potentially leading to tubular dysfunction and electrolyte imbalances. The downregulation of ACE2 receptors by the virus may disrupt the renin-angiotensin-aldosterone system (RAAS), resulting in unopposed angiotensin II activity, increased sodium reabsorption and subsequent hypernatremia. Additionally, COVID-19-related hypophysitis can impair antidiuretic hormone (ADH) secretion, leading to central diabetes insipidus and exacerbating hypernatremia.
Central mechanisms
COVID-19 can directly affect the central nervous system (CNS) through various routes, including hematogenous dissemination and neuronal invasion. ACE2 receptors in the hypothalamus and pituitary gland suggest potential viral tropism for these structures. Viral-mediated damage to the hypothalamus-pituitary axis may disrupt ADH synthesis and release, contributing to the development of central diabetes insipidus and hypernatremia. Moreover, extrapontine osmotic demyelination, a rare complication associated with the rapid correction of chronic hyponatremia, can further exacerbate neurological manifestations in patients with COVID-19-associated hypernatremia.
Clinical implications
Effective management of hypernatremia in patients with COVID-19 requires a multifaceted approach. Monitoring fluid balance, electrolyte levels and urine output is essential for early detection and intervention. Correction of hypernatremia should be gradual to prevent osmotic demyelination syndrome and minimize neurological sequelae. In refractory cases, treatment with desmopressin may be necessary to restore ADH function and improve water reabsorption. Additionally, neuroimaging studies such as magnetic resonance imaging (MRI) can provide valuable insights into the underlying etiology of hypernatremia and guide appropriate management strategies.
Figure 1
Figure A. Brain magnetic resonance image showing the absence of hyperintensity in T1 (red circle)
Limitations and strengths
The main limitation of this case report is its retrospective nature, which precludes the establishment of causal relationships between COVID-19 infection and the development of hypernatremia. Additionally, the absence of histopathological confirmation of viral infiltration in the hypothalamus and pituitary gland limits the definitive diagnosis of COVID-19-associated hypophysitis. Furthermore, the patient's comorbidities, such as arterial hypertension and the use of angiotensin II receptor antagonists, may have contributed to the pathophysiology of hypernatremia, confounding the interpretation of causal associations. Strengths of this report include the detailed clinical presentation, diagnostic workup, and neuroimaging findings, which provide valuable insights into the mechanisms underlying hypernatremia in patients with severe COVID-19. Additionally, the successful management of hypernatremia with desmopressin highlights the importance of early recognition and targeted treatment of central diabetes insipidus in this population. Despite these limitations, this case underscores the need for further research to elucidate the pathophysiology and optimal management of electrolyte disturbances in patients with COVID-19.
CONCLUSION
This case report underscores the importance of considering hypernatremia as a potentially serious complication in patients with severe COVID-19 infection. The variety of underlying mechanisms, including renal dysfunction and alterations in the hypothalamic-pituitary axis, highlight the complexity of its management. Rapid identification and treatment of central diabetes insipidus associated with COVID-19 are crucial for improving clinical outcomes and reducing associated morbidity. Further studies are needed to fully understand the risk factors and optimal management strategies for hypernatremia in this clinical context.